Concomitant coronary artery disease (CAD) is prevalent among aortic stenosis patients; however the optimal therapeutic strategy remains debated. We investigated periprocedural outcomes among patients undergoing transcatheter aortic valve implantation with percutaneous coronary intervention (TAVI/PCI) vs surgical aortic valve replacement with coronary artery bypass grafting (SAVR/CABG) for aortic stenosis with CAD.
MethodsUsing discharge data from the Spanish National Health System, we identified 6194 patients (5217 SAVR/CABG and 977 TAVI/PCI) between 2016 and 2019. Propensity score matching was adjusted for baseline characteristics. The primary outcome was in-hospital all-cause mortality. Secondary outcomes were in-hospital complications and 30-day cardiovascular readmission.
ResultsMatching resulted in 774 pairs. In-hospital all-cause mortality was more common in the SAVR/CABG group (3.4% vs 9.4%, P <.001) as was periprocedural stroke (0.9% vs 2.2%; P=.004), acute kidney injury (4.3% vs 16.0%, P <.001), blood transfusion (9.6% vs 21.1%, P <.001), and hospital-acquired pneumonia (0.1% vs 1.7%, P=.001). Permanent pacemaker implantation was higher for matched TAVI/PCI (12.0% vs 5.7%, P <.001). Lower volume centers (< 130 procedures/y) had higher in-hospital all-cause mortality for both procedures: TAVI/PCI (3.6% vs 2.9%, P <.001) and SAVR/CABG (8.3 vs 6.8%, P <.001). Thirty-day cardiovascular readmission did not differ between groups.
ConclusionsIn this large contemporary nationwide study, percutaneous management of aortic stenosis and CAD with TAVI/PCI had lower in-hospital mortality and morbidity than surgical intervention. Higher volume centers had less in-hospital mortality in both groups. Dedicated national high-volume heart centers warrant further investigation.
Keywords
Transcatheter aortic valve implantation (TAVI) has now become an established treatment for symptomatic severe aortic stenosis (AS). Multiple studies have demonstrated comparable, and in some instances, improved outcomes with TAVI than with surgical aortic valve replacement (SAVR), regardless of surgical risk.1 Coexisting coronary artery disease requiring revascularization is present in up to 20% of patients with severe AS with the prevalence of previous revascularization by either percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) in this cohort being much higher.2–4 However, doubt remains regarding the optimal treatment for these patients, who have been excluded from most randomized trials. Recent data have suggested that PCI is safe prior to TAVI with low rates of target lesion and target vessel failure at 2 years.5 On the other hand, incomplete revascularization or high residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score confers an increased risk of major adverse cardiovascular and cerebrovascular events.5,6 Current guidelines suggest that both SAVR/CABG or TAVI/PCI may be appropriate in patients undergoing aortic valve replacement procedures but in the context of concomitant complex coronary artery disease, SAVR and CABG may be favored.7,8 However, head-to-head comparisons of TAVI/PCI vs SAVR/CABG are scarce. Recent meta-analyses of published studies to date have suggested comparable short-term outcomes between patients undergoing TAVI and PCI vs SAVR and CABG, but the included studies were small and with a high risk of bias.9,10 Furthermore, many studies on this important clinical question have included patients from the time when TAVI was first becoming recognized as a viable treatment for AS and may no longer reflect contemporary practice, current generation devices, and indeed, outcomes. As such, we aimed to assess contemporary outcomes in patients undergoing PCI and TAVI vs SAVR with concomitant CABG in a nationwide population.
METHODSStudy populationThis was a retrospective observational study of all patients discharged from hospitals within the Spanish National Health System following surgical aortic valve replacement (SAVR) or TAVI with associated coronary revascularization from January 2016 to December 2019. Coronary revascularization was via CABG in those undergoing SAVR, and PCI in those undergoing TAVI. Revascularization occurred within the same episode of care for those undergoing SAVR, and within the 6 months prior to TAVI in those undergoing a percutaneous procedure.
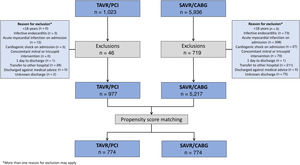
Data were extracted from the minimum data set of the Spanish National Health System using the International Classification of Diseases 10th edition (ICD-10). ICD-10 codes used to extract data on procedures, comorbidities and complications are shown in table 1 of the supplementary data. Multiple hospitalizations resulting from transfers between hospitals were considered as a single care episode, which was attributed in this study to the most complex hospital. Patients were excluded if they were <18 years, were discharged against medical advice, or had a length of stay of 1 day or less before being discharged alive to home. Patients presenting initially with infective endocarditis, cardiogenic shock or acute myocardial infarction were also excluded, as were those with a concomitant mitral or tricuspid intervention. Finally, patients undergoing TAVI and PCI within the same care episode (same hospital admission) were excluded as it was not possible to determine if these were planned or rescue (bail out) procedures due to coronary obstruction or other procedural complications.
The primary outcome was in-hospital all-cause mortality. Secondary outcomes comprised periprocedural complications: acute kidney injury, periprocedural myocardial infarction, periprocedural stroke, permanent pacemaker (PPM) implantation, length of hospital stay, and 30-day cardiovascular readmission. As a secondary analysis, we also examined the impact of hospital procedural volume on outcomes. Given the retrospective nature of the study and the administrative data used, the need to obtain individual informed consent was waived.
Statistical analysisCategorical variables are expressed as number and percentage while continuous variables are expressed as mean and standard deviation (SD) or median and interquartile range [25th-75th percentile] according to their distribution. Qualitative variables were analyzed using the chi-square or Fisher exact test and differences in continuous variables were analyzed using a 2-sided Student t test or Mann Whitney U test according to their distribution for the unmatched comparison.
Multilevel logistic regression models were specified and adjusted for all-cause in-hospital mortality, based on the methodology of the Centers for Medicare and Medicaid Services for coronary artery bypass grafting, adapted to the data structure of the minimum data set, after the secondary diagnoses were grouped according to the condition categories updated yearly by the Agency for Healthcare Research and Quality.11,12 Variables included were those baseline characteristics found to be statistically significant on univariable analysis with an odds ratio (OR)> 1.00. Backward elimination regression was then performed with significance for inclusion being P <.05 and for elimination being ≥ 0.10. In-hospital risk-adjusted all-cause mortality ratios were calculated from these specified models. Calibration was analyzed graphically after patients were grouped in deciles with respect to the predicted probabilities, and tabulation of the mean predicted vs observed probabilities. Discrimination was assessed by the area under the receiver operating characteristic curves (AUROC).
To minimize bias due to differences in baseline characteristics between the 2 groups, propensity score matching was performed to assess the impact of TAVI with PCI vs SAVR with CABG on in-hospital all-cause mortality. Matching was performed using psmatch2 option of Stata v16 with a logistic regression model (option logit) and match on the OR (option odds), a 1:1 ratio and a ‘nearest neighbour’ match and a caliper of 0.05 without replacement. Among the episodes with TAVI and PCI, we selected those with a profile more similar to each episode of SAVR and CABG, according to the variables that were statistically significant in the risk-adjustment models.
The probability of in-hospital death, the effect of differences between groups (average treatment effect) and OR with 95% confidence intervals (95%CI) were calculated. Assessment of the appropriateness of the matching was performed by constructing Kernel density plots to graphically represent the populations before and after matching and by calculation of the standardized mean differences for all covariates. Comparison of continuous and categorical variables between the matched groups were as previously described for unmatched groups. A P value of <.05 was considered statistically significant. A sensitivity analysis was performed to estimate whether there was a selection bias within the TAVI/PCI group as patients undergoing PCI who died before undergoing TAVI were not included. A worst-case scenario analysis was performed (compared with our original analysis) by calculating the crude all-cause mortality rate for patients with a diagnosis of AS undergoing PCI (AS/PCI) during the study period who were not in either of the study groups (TAVI/PCI and SAVR/CABG). These patients were matched by propensity scores with patients in the TAVI/PCI group and the crude mortality rate was calculated. Finally, we compared all-cause in-hospital mortality rates between “low volume” and “high-volume” centers. To discriminate between high- and low-volume centers a K-means clustering algorithm was used, with the aim of obtaining the maximal intragroup and the minimal intergroup density. The mathematical model was constructed with two thirds of the dataset and was validated with the remaining one third. We calculated a cutoff point to define “low-volume” and “high-volume” centers, and the impact of procedure volume on in-hospital all-cause mortality was determined. All statistical analyses were performed using Stata 16 (StataCorp, College Station, Texas, USA) and Statistical Package for Social Science (SPSS) version 21.0.
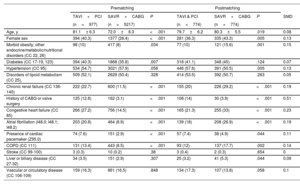
RESULTSFrom January 2016 to December 2019, 6194 patients were identified as meeting the inclusion criteria (977 TAVI and PCI vs 5217 SAVR and CABG) (figure 1). While all CABG procedures were performed during the same episode of care as SAVR procedures, the median time between PCI and TAVI was 61 [IQR 30-107] days. Several baseline characteristics differed between the groups. TAVI patients were older (81.1 vs 72 years, P <.001), were more commonly female (40.3 vs 26.4%, P <.001) and more commonly had a history of heart failure, prior CABG, chronic renal failure, atrial fibrillation, and chronic obstructive pulmonary disease (P <.001 for all comparisons). table 1 summarizes the baseline characteristics of the unmatched and matched cohorts.
Baseline characteristics of the matched and unmatched cohorts
| Prematching | Postmatching | ||||||
|---|---|---|---|---|---|---|---|
| TAVI+PCI | SAVR+CABG | P | TAVI & PCI | SAVR+CABG | P | SMD | |
| (n=977) | (n=5217) | (n=774) | (n=774) | ||||
| Age, y | 81.1± 6.3 | 72.0±8.3 | <.001 | 79.7±6.2 | 80.3±5.5 | .019 | 0.08 |
| Female sex | 394 (40.3) | 1377 (26.4) | <.001 | 281 (36.3) | 335 (43.3) | .005 | 0.13 |
| Morbid obesity; other endocrine/metabolic/nutritional disorders (CC 22, 26) | 98 (10) | 417 (8) | .034 | 77 (10) | 121 (15.6) | .001 | 0.15 |
| Diabetes (CC 17-19, 123) | 394 (40.3) | 1868 (35.8) | .007 | 318 (41.1) | 348 (45) | .124 | 0.07 |
| Hypertension (CC 95) | 534 (54.7) | 3021 (57.9) | .058 | 446 (57.6) | 391 (50.5) | .005 | 0.13 |
| Disorders of lipoid metabolism (CC 25), | 509 (52.1) | 2629 (50.4) | .328 | 414 (53.5) | 392 (50.7) | .263 | 0.05 |
| Chronic renal failure (CC 136-140) | 222 (22.7) | 600 (11.5) | <.001 | 155 (20) | 226 (29.2) | <.001 | 0.19 |
| History of CABG or valve surgery | 125 (12.8) | 162 (3.1) | <.001 | 108 (14) | 30 (3.9) | <.001 | 0.51 |
| Congestive heart failure (CC 85) | 266 (27.2) | 756 (14.5) | <.001 | 165 (21.3) | 255 (33) | <.001 | 0.23 |
| Atrial fibrillation (I48.0; I48.1; I48.2) | 203 (20.8) | 464 (8.9) | <.001 | 139 (18) | 208 (26.9) | <.001 | 0.19 |
| Presence of cardiac pacemaker (Z95.0) | 74 (7.6) | 151 (2.9) | <.001 | 57 (7.4) | 38 (4.9) | .044 | 0.11 |
| COPD (CC 111) | 131 (13.4) | 443 (8.5) | <.001 | 93 (12) | 137 (17.7) | .002 | 0.14 |
| Stroke (CC 99-100) | 3 (0.3) | 10 (0.2) | .38 | 3 (0.4) | 2 (0.3) | .654 | 0 |
| Liver or biliary disease (CC 27-32) | 34 (3.5) | 151 (2.9) | .307 | 25 (3.2) | 41 (5.3) | .044 | 0.09 |
| Vascular or circulatory disease (CC 106-109) | 159 (16.3) | 861 (16.5) | .848 | 134 (17.3) | 107 (13.8) | .058 | 0.1 |
CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention; SAVR, surgical aortic valve replacement; SMD, standardized mean difference; TAVI, transcatheter aortic valve implantation; TIA, transient ischemic attack.
Values are expressed as No. (%) or mean±standard deviation.
CC: Condition categories. Secondary diagnoses grouped in risk factors (Pope et al).11,12
Propensity score matching resulted in 774 pairs. The variables included in the matching were age, female sex, hypertension, disorders of lipid metabolism, diabetes, morbid obesity, other endocrine/metabolic/nutritional disorders, chronic renal failure, history of CABG or valve surgery, congestive heart failure, atrial fibrillation, presence of cardiac pacemaker, chronic obstructive pulmonary disease, stroke, liver, or biliary disease, and vascular or circulatory disease. Kernel density plots before and after matching are depicted in figure 2A, B. Although a close match was achieved, some differences remained. TAVI patients more commonly had a history of prior cardiac surgery (14.0 vs 3.9%; P <.001), hypertension (57.6 vs 50.5%; P=.005), and PPM (7.4 vs 4.9%; P=.044). However, the proportion of some variables (such as heart failure and chronic renal failure) were inverted between groups before and after the pairing, being more frequent in the SAVR cohort after the propensity match.
Kernel Density plots representing pre- (A) and post- (B) matching. Patients were matched based on the following variables: age, sex, diabetes, obesity, chronic obstructive pulmonary disease, previous history of atrial fibrillation, previous stroke, renal failure, heart failure, liver disease and respiratory failure at presentation. SAVR/CABG, surgical aortic valve replacement with coronary artery bypass grafting; TAVI/PCI, transcatheter aortic valve implantation with percutaneous coronary intervention.
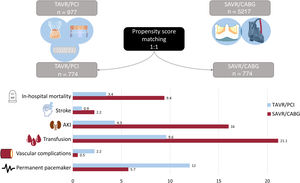
table 2 summarizes the in-hospital outcomes between the 2 cohorts. The primary endpoint (crude in-hospital all-cause mortality) was lower in the matched TAVI group vs SAVR (average treatment effect: 3.4% vs 9.4%; OR=0.34; 95%CI, 0.20-0.54; P <.001) (figure 3). After application of the sensitivity analysis, a crude all-cause mortality rate of 3.4% was obtained for patients with a diagnosis of AS undergoing PCI (AS/PCI) during in the study period who were not in either of the study groups. If this crude mortality rate were added to the average treatment effect of the TAVI group calculated in the original analysis (3.4%, best-case) a worst-case all-cause mortality of 6.8% would be obtained vs the SAVR/CABG average treatment effect: 9.4%, P <.005. Other in-hospital complications such as periprocedural stroke (0.9% vs 2.2%; P=.004), requirement for blood transfusion (9.6% vs 21.1%), acute kidney injury (4.3% vs 16.0%; P <.001), pericardial complications (1.4% vs 3.0%; P=.037), and hospital-acquired pneumonia (0.1% vs 1.7%; P=.001) were also more frequent in the SAVR group. Vascular complications (2.2% vs 0.5%; P=.004) and the need for in-hospital PPM implantation was higher in TAVI patients (12.0% vs 5.7%; P <.001). Length of stay, both in the intensive care unit and in-hospital overall, was significantly longer in those patients undergoing SAVR/CABG than in those undergoing TAVI/PCI. Data on 30-day cardiovascular readmission were available for all patients and did not differ between groups.
Postprocedure clinical endpoints for TAVI/PCI and SAVR/CABG cohorts
| Prematching | Postmatching | |||||
|---|---|---|---|---|---|---|
| Clinical endpoints | TAVI & PCI(n=977) | SAVR & CABG(n=5217) | P | TAVI & PCI(n=774) | SAVR & CABG(n=774) | P |
| In-hospital all-cause mortality | 3.0 | 7.0 | <.001 | 3.4 | 9.4 | <.001 |
| Periprocedural stroke (CC100) | 1.0 | 1.7 | .103 | 0.9 | 2.2 | .04 |
| Acute kidney injury (CC135) | 4.7 | 10.3 | <.001 | 4.3 | 16.0 | <.001 |
| Blood transfusion | 11.1 | 19.1 | <.001 | 9.6 | 21.1 | <.001 |
| Pericardial complicationsa | 1.5 | 3.5 | .001 | 1.4 | 3.0 | .037 |
| Acute myocardial infarction (CC86) | 0.4 | 1.4 | .013 | 0.5 | 1.2 | .164 |
| Vascular complicationsb | 3.0 | 0.6 | <.001 | 2.2 | 0.5 | .004 |
| Aspiration and specified bacterial pneumonias (CC114) | 0.2 | 1.2 | .006 | 0.1 | 1.7 | .001 |
| New permanent pacemaker implantationc | 13.0 | 3.5 | <.001 | 12.0 | 5.7 | <.001 |
| New onset atrial fibrillation | 1.3 | 2.8 | .007 | 1.0 | 2.5 | .033 |
| Length of stay in intensive care | 0 [0-1] | 2 [0-4] | <.001 | 0 [0-1] | 2 [0-4] | <.001 |
| Length of hospital stay (days) | 7 [6-10] | 12 [9-20] | <.001 | 7 [5-10] | 14 [9-23] | <.001 |
| 30-day cardiovascular readmission | 7.55 | 7.8 | 0.63 | 6.4 | 6.8 | .810 |
CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Values are expressed as No. (%) or median [interquartile range].
Pericardial complications including pericarditis, pericardial effusion, hemopericardium, cardiac tamponade, and the need for pericardiocentesis or pericardial window.
Calculated only for patients without pre-exiting permanent pacemakers.
CC: Condition categories. Secondary diagnoses grouped on risk factors (Pope et al).11
Central illustration. In-hospital complications for patients undergoing TAVI/PCI vs those undergoing SAVR/CABG. AKI, acute kidney injury; SAVR/CABG, surgical aortic valve replacement with coronary artery bypass grafting; TAVI/PCI, transcatheter aortic valve implantation with percutaneous coronary intervention.
The multivariable logistic regression model used for in-hospital all-cause mortality demonstrated acceptable discrimination with an AUROC of 0.70. When procedures were included in this model, SAVR had a higher risk of in-hospital all-cause mortality than TAVI (OR=3.1; 95%CI, 2.1-4.5; P <.001).
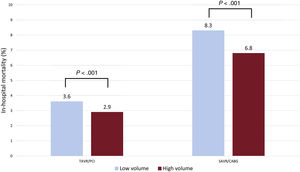
We also investigated whether procedure volume impacted on in-hospital all-cause mortality. Using a clustered algorithm with K-means, a cutoff of 130 procedures per year for both TAVI/PCI and SAVR/CABG was optimum for demonstrating a difference in the primary outcome. Those centers performing <130 procedures per year demonstrated an increased risk of in-hospital risk-adjusted all-cause mortality: TAVI/PCI 3.6% vs 2.9%; P <.001 and SAVR/CABG 8.3% vs 6.8%; P <.001 for low- and high-volume centers respectively (figure 4).
Differences in in-hospital all-cause mortality for TAVI/PCI and SAVR/CABG by center volume (low <130, high ≥ 130 procedures per year). SAVR/CABG, surgical aortic valve replacement with coronary artery bypass grafting; TAVI/PCI, transcatheter aortic valve implantation with percutaneous coronary intervention.
Our study presents the results from a large propensity matched population of symptomatic patients with AS and severe coronary artery disease undergoing either entirely percutaneous (TAVI and PCI) or entirely surgical (concomitant SAVR and CABG) management. The main findings are as follows: a) in-hospital all-cause mortality was more frequent among SAVR/CABG patients than TAVI/PCI patients with an OR of 3.1; b) periprocedural complications including periprocedural stroke, requirement for blood transfusion, acute kidney injury, pericardial complications and hospital-acquired pneumonia were all more common in the SAVR/CABG group; c) rates of PPM implantation prior to discharge were twice as high in the TAVI/PCI cohort than the SAVR/CABG cohort; d) procedure volume significantly impacted in-hospital all-cause mortality in both (percutaneous and surgical) groups.
Coronary artery disease is highly prevalent among patients presenting with symptomatic severe AS and coronary revascularization continues to present a clinical dilemma in the choice between TAVI and SAVR. Indeed, even the indication for revascularization may be difficult to ascertain with certainty, due to considerable overlap between the symptoms of coronary artery disease and those of severe AS. Nonetheless, current guidelines recommend revascularization for stenosis ≥ 70% in proximal segments, and an almost 10-fold increase in percutaneous revascularization prior to TAVI has been reported.7,13 With repeatedly demonstrated equivalent outcomes between TAVI and SAVR, the concept of an entirely percutaneous approach in the presence of coronary artery disease presents an attractive, less invasive option. Registry data support a percutaneous approach with good outcomes for patients undergoing PCI prior to TAVI regardless of anatomical complexity.5 Direct comparisons of TAVI/PCI vs SAVR/CABG are, however, scarce. The only randomized data available to date on this topic is provided by the SURTAVI trial.14 In that study, a subgroup of patients with coronary artery disease (SYNTAX score ≤ 22 without left main disease) were randomized to revascularization by either PCI, in those undergoing TAVI, or CABG, in those undergoing SAVR, and no differences in all-cause mortality or stroke were seen at 30-days or 2 years. Similarly, a post hoc analysis of the PARTNER 3 study demonstrated no difference in the composite endpoint of death from any cause, stroke or rehospitalization (hazard ratio=0.52; 95%CI, 0.11-2.49) in a small subgroup of patients undergoing TAVI with concomitant PCI (n=32) vs SAVR with CABG (n=58)2. Many of the remaining data come from observational registry studies. In a propensity matched analysis of the OBSERVANT registry, Barbanti et al.15 found no difference in early mortality in those undergoing TAVI/PCI vs SAVR/CABG and, similarly, an analysis of the National Inpatient Sample database in the US between 2012 and 2017 again found no difference in in-hospital mortality between groups,13 although a more recent analysis of this database including only those treated between 2016 and 2017 demonstrated a lower adjusted OR for in-hospital mortality in the TAVI/PCI group (OR=0.32; 95%CI, 0.17-0.62; P=.001).16 More recently, in the context of complex coronary artery disease (left main stem or SYNTAX score> 22), no difference in major adverse cardiovascular or cerebrovascular events at 3 years was found between those undergoing TAVI/PCI vs SAVR/CABG, although a higher rate of new revascularization was seen in the TAVI/PCI group.17
Unlike these studies, our study found increased in-hospital all-cause mortality in patients undergoing SAVR/CABG vs TAVI/PCI. One explanation could be that, in our study, percutaneous revascularization was performed as a staged procedure prior to TAVI, allowing patients time to recover, while the previously cited studies included concomitant TAVI/PCI in some, but not all, patients. Additionally, in our study, patients who underwent PCI and died prior to undergoing TAVI were not included. However, in our sensitivity analysis, which attempted to account for this, TAVI/PCI continued to have lower in-hospital all-cause mortality vs SAVR/CABG when taking “worst-case scenario” mortality rates into account. Lastly, particularly in retrospective observational studies, PCI may have been performed as a complication of TAVI rather than as a planned procedure and may explain the absence of mortality difference between the groups compared with our study, which excluded patients who underwent PCI and TAVI in the same care episode to exclude those that had PCI as a result of a complication or as a ‘rescue’ treatment. The subanalysis of the National Inpatient Sample database by Patlolla et al.13 would support a treatment strategy of staged percutaneous procedures. In that study, patients who underwent staged PCI followed by TAVI had reduced in-hospital mortality compared with those undergoing same day TAVI/PCI. Conversely, concomitant SAVR with CABG is carried out simultaneously resulting in longer procedure times, and time on cardiopulmonary bypass, which may partly explain the increased in-hospital all-cause mortality seen in the SAVR/CABG group in our study. Although our study contributes important data on this issue, specific prospective randomized studies are clearly required to further compare TAVI/PCI with SAVR/CABG. The currently recruiting TCW trial (NCT03424941) aims to examine this issue using a more nuanced approach to percutaneous revascularization with prior fractional flow reserve assessment and the use of newer generation devices.
Acute kidney injury was higher in the SAVR/CABG group than in the TAVI/PCI group, which has been a consistent finding in all TAVI vs SAVR studies to date, with and without revascularization.9,18–20 The ability to stage revascularization prior to the TAVI procedure can result in reduced nephrotoxic agents (contrast) with less subsequent kidney injury and more time for renal function recovery between procedures. When given the choice, staging PCI and TAVI procedures appears to be the preferred option for many physicians, with both the SURTAVI study14 and that of Barbanti et al.15 having predominantly staged PCI procedures (59% and 92% respectively), despite no prespecification for staging (in fact in the SURTAVI study, concomitant TAVI and PCI procedures were encouraged). Subsequently, acute kidney injury rates in these studies were similar to those in our present study. Staging CABG and SAVR procedures, however, is not recommended and concomitant SAVR/CABG are longer, with longer cardiopulmonary bypass times being previously associated with a higher incidence of acute kidney injury.21 This may be a particularly important consideration in patients with chronic kidney disease, a prevalent comorbidity in patients with symptomatic AS (and present in ∼20% of our cohort). The importance of avoiding periprocedural acute kidney injury cannot be over emphasized, with acute kidney injury being consistently found as a predictor of both short- and long-term mortality in both TAVI and SAVR populations with and without pre-existing renal dysfunction.20–23 The lower incidence of this complication in the TAVI/PCI population is therefore an important consideration when choosing between purely percutaneous and surgical strategies in patients with severe AS and concomitant coronary artery disease, with TAVI/PCI offering some advantages in this regard.
Requirement of PPM continues to be a hurdle for TAVI, and our study again demonstrated an increase in PPM requirement among the TAVI population compared with SAVR. However, it is evident that the rate of PPM following TAVI has been steadily decreasing and this is reflected in the lower PPM requirement in our study than that of the SURTAVI trial, and similar to the more recent study by Barbanti.14,15 One likely explanation is that our study included all types of available transcatheter valves and more contemporary patients (inclusion from 2016-2019) compared with the SURTAVI trial, which included only self-expanding valves and recruited patients from 2012-2016. Recent advances in implantation techniques for TAVI particularly with self-expanding valves, has resulted in decreased pacemaker requirement in more contemporary studies, and adaption of these techniques across centers may explain the lower PPM rates seen in our study.
In this study, center experience reflected in procedure volume demonstrated an impact on in-hospital all-cause mortality. For both TAVI/PCI and SAVR/CABG, a procedure volume <130 per year was associated with increased in-hospital all-cause mortality compared with centers performing ≥ 130 procedures per year. The association between procedure volume and outcomes has been previously demonstrated in a meta-analysis by He et al., and analyses of the TVT registry.24–26 Similar to our study, reduced in-hospital and 30-day mortality, as well as other periprocedural complications such as bleeding and stroke have been found with increased procedural volume in these aforementioned studies.24–26 Patients requiring both coronary revascularization and treatment of severe AS are inherently complex, making these findings unsurprising. They do highlight, however, the potential beneficial impact on outcomes of high-volume, specialized heart centers with combined surgical and interventional skills acting as tertiary referral centers and this should be a point of discussion and further research.
LimitationsThis study was carried out using data from a National Health System database and has the inherent limitations pertaining to this study design. Hence, some individual patient data were not available, including data on imaging, laboratory tests and specific anatomical data relating to patients’ coronary artery disease (eg, SYNTAX score) and aortic root anatomy. These missing variables could not be sought and may introduce a selection bias regarding the treatment decision, despite propensity matching. The accuracy of the data relies on adequate hospital coding, and it is prone to underestimate some complications. Furthermore, patients dying during or after their PCI procedure and before the planned TAVI were not included in the crude all-cause mortality calculations and therefore the all-cause mortality rate in the TAVI/PCI arm may be underestimated. However, the specific features of the interventions performed, and the endpoint of in-hospital all-cause mortality made incorrect coding for the primary outcome very unlikely compared with other in-hospital complications. While propensity score matching aims to minimize differences between cohorts, some differences remained. Furthermore, the presence of unidentified confounding factors cannot be excluded, and we cannot rule out the possibility that these affected the results. Future confirmatory studies are therefore required. In addition, long-term follow-up was not available for the included patients and so our results are limited to in-hospital complications and 30-day cardiovascular readmissions. Thus, we could not assess the potential advantages of surgical intervention in younger patients at long-term follow-up.
CONCLUSIONSIn a large contemporary national database, patients with symptomatic severe AS and concomitant coronary artery disease requiring revascularization, percutaneous intervention (TAVI/PCI) resulted in decreased in-hospital all-cause mortality, periprocedural stroke, blood transfusion requirement, acute kidney injury, and hospital-acquired pneumonia than SAVR with concomitant CABG. Patients in the TAVI cohort had an increased requirement for PPM implantation. In both procedures, greater center experience was associated with reduced in-hospital all-cause mortality. These findings are important considerations for the cardiology community managing patients with AS and concomitant coronary artery disease and suggest the potential usefulness of an entirely percutaneous approach in these complex patients.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSA. McInerney, M. García-Marquez, J.L. Bernal, C. Fernandez-Perez, J. Elola, L. Nombela-Franco: substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work, drafting the work or revising it critically for important intellectual contente and final approval of the version to be published;
G. Tirado-Conte, P. Jimenez-Quevedo, N. Gonzalo, I. Nuñez-Gil, N. Prado, J. Escaned, A. Fernández-Ortiz: drafting the work or revising it critically for important intellectual contente and final approval of the version to be published.
CONFLICTS OF INTERESTL. Nombela-Franco has received consulting fees from Edwards Lifesciences and Abbott Vascular. The other authors have no relationships to disclose.
- –
Coronary artery disease is present in ∼20% of patients presenting with symptomatic severe AS.
- –
There are limited data comparing surgical (SAVR and CABG) vs percutaneous (TAVI and PCI) strategies in these patients and, as a result, the optimal management is still debated.
- –
In this propensity score matched analysis, staged TAVI/PCI resulted in reduced in-hospital all-cause mortality compared with concomitant surgical aortic valve replacement and coronary artery bypass grafting (SAVR/CABG).
- –
Other periprocedural complications including stroke, blood transfusions, acute kidney injury, pericardial complications, and hospital-acquired pneumonia were more common in the SAVR/CABG group, while new permanent pacemaker implantation was more frequent in the TAVI/PCI group.
- –
These results suggest that a percutaneous approach to concomitant AS and coronary artery disease can result in both reduced periprocedural morbidity, and mortality.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.12.011