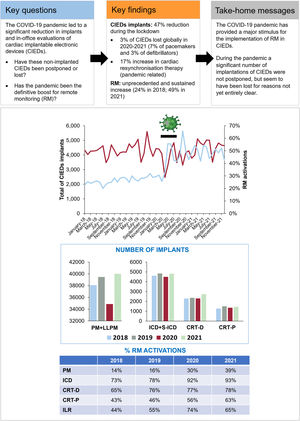
Remote monitoring (RM) of cardiac implantable electronic devices (CIEDs) is considered more reliable, efficient, and safer than conventional in-person follow-up. However, the implementation of RM is still suboptimal. This study aimed to analyze the impact of the COVID-19 pandemic on the rates of CIED implants and RM activations in Spain.
MethodsThe COVID-19 RM Spain Registry was used to analyze the monthly number of all CIED implantations and RM activations from January 2018 to December 2021. A descriptive analysis was performed using aggregated data from the five major CIED manufacturers.
ResultsA total of 205 345 CIEDs were recorded. The number of implants decreased sharply (48.2%) during the pandemic lockdown (March-June 2020) but gradually increased thereafter, compensating for the previous reduction. However, pacemakers and implantable cardiac defibrillators (ICD) showed an aggregate loss of 7% and 3%, respectively, from the annual average during 2020-2021. In contrast, cardiac resynchronization therapy defibrillators (CRT-D) increased by 17%, and pacemakers (CRT-P) by 4.5% over the 2-year period. The percentage of RM activations increased from 24.5% in 2018 to 49.0% in 2021, with a sharp increase during the lockdown. The RM activation rates consistently increased during the lockdown for all devices: pacemakers (14.4% vs 37.2%; P <.001); ICD (75.6% vs 94.2%; P <.001); CRT-D/CRT-P (68.6-44.2% vs 81.6-61%; P <.001), and implantable loop recorders (50.2% vs 68.7%; P <.001).
ConclusionsThe significant decline in implants during the lockdown gradually recovered, except for pacemakers and ICD. However, the COVID-19 pandemic boosted RM for all CIEDs in Spain.
Keywords
Remote monitoring (RM) has proven to be a reliable, safe, and cost-effective method for monitoring for most cardiac implantable electronic devices (CIEDs)1–7 and may increase survival.8–11
Therefore, RM is recommended for monitoring all CIEDs starting almost immediately after implantation.12 Despite the benefits, there are some barriers to its widespread implementation, such as legal issues, lack of reimbursement,13 lack of recognition by health authorities and, derived from the latter, limited provision of human and material resources. Data from a registry of a single US manufacturer of over 300 000 CIEDs showed that 53% of them lacked RM.10 European surveys assessing the implementation of RM have involved few centers, but the data show adoption rates of 14% for pacemakers, 51% for implantable cardioverter-defibrillators (ICD), and 49% for cardiac resynchronization therapy (CRT, including CRT-P [pacemakers] and CRT-D [defibrillators]) devices.13,14
During the COVID-19 pandemic in Spain, a general lockdown was decreed in an attempt to address the massive pressure on health care systems (March 15-June 21, 2020). During this period, an increase in sudden death was observed, as well as a reduction in resuscitation attempts,15,16 and a significant reduction in cardiac procedures, including CIEDs implantations.17–22 Regarding device follow-ups, the lockdown resulted in the cancellation of all nonurgent in-person evaluations (IPE). In the postlockdown period, IPE were greatly limited and the periods between visits were significantly further apart.
As a consequence, most scientific societies recommended the use of RM instead of IPE in the follow-up of CIED carriers.23–25 Many institutions followed these recommendations and some have published their experience in CIED management during the pandemic.26 A recent survey promoted by the European Heart Rhythm Association (EHRA) collected data from 160 institutions in 28 countries (50% of them from France and Spain), which voluntarily reported their percentage of pre- and postpandemic RM. The survey found an increase in RM use in all implantable devices, but these increases were statistically significant only for pacemakers and implantable loop recorders (ILR).27 The pandemic may have accelerated the acceptance and adoption of RM, even in countries without reimbursement for RM.28
The COVID-19 RM Spain Registry is a survey conceptualized by the principal investigator and supported by the Heart Rhythm Association of the Spanish Society of Cardiology, which aimed to quantify the differences in the percentage of RM activations of all CIEDs before, during and after the lockdown, both regionally and countrywide. In addition, the study analyzed variations in the implantation rate of CIEDs in this period.
METHODSStudy designA request for information was submitted in April 2020 to the main CIED manufacturers (Abbott Medical, United States; Biotronik SE & Co, Germany; Boston Scientific, United States; Medtronic, United States; and MicroPort CRM, China). These companies provided information on all pacemakers, ICD (single or dual chamber), CRT-P, CRT-D, ILR, subcutaneous ICD (S-ICD), and leadless pacemakers (LLPM) invoiced, as well as the number of devices activated in each of their proprietary RM systems from January 2018 to December 2021 in each region of Spain (all data were anonymized, and the principal investigator was the only person who had access to each manufacturer's data). Data were requested or accessed at the regional level, but not from specific hospitals. Data on the population was obtained from the National Institute of Statistics (INE). The registry was approved by the ethics committee of the coordinating center (Burgos University Hospital).
Data reliabilityTo the best of our knowledge, there is currently no accurate benchmark for remote monitoring (RM) activations of CIEDs. Therefore, in this study, the only externally benchmarked data were the numbers of implants. Comparison of these implant numbers with data provided by Medtech Europe (Medical Technology industries) and national implant registries revealed a significant similarity. However, variations in rates per population were observed due to the use of different sources of demographic information.29–31
Statistical analysisContinuous variables are presented as counts or median (interquartile range [IQR]). Categorical variables are presented as frequencies and percentages. Joinpoint regression models were used for trend analysis using the Program software (Version 4.9.0.0. March21; Statistical Research and Applications Branch, National Cancer Institute, United States). These models were used to identify the time at which significant changes in the trend occurred and to estimate the magnitude of the observed increase or decrease in each interval. Thus, the months (period) comprising each trend, as well as the monthly percentage change and their confidence intervals for each trend, were expressed in the results. The value of MPC (monthly percentage change) was considered statistically significant if it differed from 0 with a P <.05.
To analyze the time difference in means of implants and percentage of RM activations, we grouped the data into 3 periods (prelockdown, lockdown and postlockdown) and evaluated if there were statistically significant differences in their rate ratios using the Stata software. Confidence intervals based on normal distribution were calculated and the exact significance was calculated with binomial probability. For the comparison of means, the Student t-test was used. P <.05 was considered statistically significant.
We also conducted an analysis to quantify the overall impact of the pandemic on device implants. To do this, we benchmarked the average number of implants from 2018 to 2019 and compared it with 2020 and 2021 respectively, thus establishing an overall estimate that reflected both the reduction in 2020 and the increase in 2021.
To define the correlation between the percentage of activations and different variables, the Pearson correlation coefficient was used. To evaluate if there was a correlation between the percentages of activations and the months that an autonomous community had been at high or very high risk for COVID-19, the Spearman rho correlation coefficient was used. Coefficients of 1, −1, or 0 indicated positive, negative, or no correlation, respectively.
Data availability statementsMost of data have been incorporated into the article and its online supplementary data. The remaining data is available on request. The data underlying this article will be shared on reasonable request to the corresponding author.
RESULTSAll centers in the country were consulted about having their data used in the registry. Therefore, all CIED implants and RM activations performed in the country were registered.
CIEDs implantationsIn 2020, the number of implants was reduced by 10.6% vs 2019, and by 8.5% vs the average for 2018 to 2019. In 2021, there was a 5% increase compared with the average for 2018 to 2019, and a 13% increase compared with 2020 (table 1). There was a sharp decline during the lockdown (48.2% on comparison of April 2020 vs April 2019) and a subsequent recovery, which did not continue the previous trend until 2021. However, the reduction and the recovery were not homogeneous for all regions or devices (table 1, table 2, figure 1 and figures 1-5 of the supplementary data).
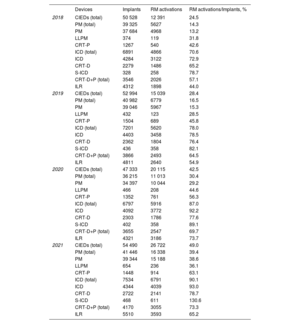
Number of CIEDs implanted and RM activations in Spain from 2018 to 2021.
| Devices | Implants | RM activations | RM activations/Implants, % | |
|---|---|---|---|---|
| 2018 | CIEDs (total) | 50 528 | 12 391 | 24.5 |
| PM (total) | 39 325 | 5627 | 14.3 | |
| PM | 37 684 | 4968 | 13.2 | |
| LLPM | 374 | 119 | 31.8 | |
| CRT-P | 1267 | 540 | 42.6 | |
| ICD (total) | 6891 | 4866 | 70.6 | |
| ICD | 4284 | 3122 | 72.9 | |
| CRT-D | 2279 | 1486 | 65.2 | |
| S-ICD | 328 | 258 | 78.7 | |
| CRT-D+P (total) | 3546 | 2026 | 57.1 | |
| ILR | 4312 | 1898 | 44.0 | |
| 2019 | CIEDs (total) | 52 994 | 15 039 | 28.4 |
| PM (total) | 40 982 | 6779 | 16.5 | |
| PM | 39 046 | 5967 | 15.3 | |
| LLPM | 432 | 123 | 28.5 | |
| CRT-P | 1504 | 689 | 45.8 | |
| ICD (total) | 7201 | 5620 | 78.0 | |
| ICD | 4403 | 3458 | 78.5 | |
| CRT-D | 2362 | 1804 | 76.4 | |
| S-ICD | 436 | 358 | 82.1 | |
| CRT-D+P (total) | 3866 | 2493 | 64.5 | |
| ILR | 4811 | 2640 | 54.9 | |
| 2020 | CIEDs (total) | 47 333 | 20 115 | 42.5 |
| PM (total) | 36 215 | 11 013 | 30.4 | |
| PM | 34 397 | 10 044 | 29.2 | |
| LLPM | 466 | 208 | 44.6 | |
| CRT-P | 1352 | 761 | 56.3 | |
| ICD (total) | 6797 | 5916 | 87.0 | |
| ICD | 4092 | 3772 | 92.2 | |
| CRT-D | 2303 | 1786 | 77.6 | |
| S-ICD | 402 | 358 | 89.1 | |
| CRT-D+P (total) | 3655 | 2547 | 69.7 | |
| ILR | 4321 | 3186 | 73.7 | |
| 2021 | CIEDs (total) | 54 490 | 26 722 | 49.0 |
| PM (total) | 41 446 | 16 338 | 39.4 | |
| PM | 39 344 | 15 188 | 38.6 | |
| LLPM | 654 | 236 | 36.1 | |
| CRT-P | 1448 | 914 | 63.1 | |
| ICD (total) | 7534 | 6791 | 90.1 | |
| ICD | 4344 | 4039 | 93.0 | |
| CRT-D | 2722 | 2141 | 78.7 | |
| S-ICD | 468 | 611 | 130.6 | |
| CRT-D+P (total) | 4170 | 3055 | 73.3 | |
| ILR | 5510 | 3593 | 65.2 |
CIEDs, cardiac implantable electronic devices; CRT-D, cardiac resynchronization therapy-defibrillators; CRT-P, cardiac resynchronization therapy-pacemakers; ICD, implantable cardiac defibrillators; ILR, implantable loop recorders; LLPM, leadless pacemakers; PM, pacemakers (single and dual chamber); S-ICD, subcutaneous implantable cardiac defibrillators.
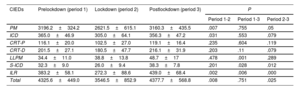
Comparison of monthly implant means of the different devices before, during and after the COVID-19 lockdown
| CIEDs | Prelockdown (period 1) | Lockdown (period 2) | Postlockdown (period 3) | P | ||
|---|---|---|---|---|---|---|
| Period 1-2 | Period 1-3 | Period 2-3 | ||||
| PM | 3196.2±324.2 | 2621.5±615.1 | 3160.3±435.5 | .007 | .755 | .05 |
| ICD | 365.0±46.9 | 305.0±64.1 | 356.3±47.2 | .031 | .553 | .079 |
| CRT-P | 116.1±20.0 | 102.5±27.0 | 119.1±16.4 | .235 | .604 | .119 |
| CRT-D | 201.5±27.1 | 180.5±47.7 | 216.1±31.9 | .203 | .11 | .079 |
| LLPM | 34.4±11.0 | 38.8±13.8 | 48.7±17 | .478 | .001 | .289 |
| S-ICD | 32.3±9.0 | 26.0±9.4 | 38.3±7.8 | .201 | .028 | .012 |
| ILR | 383.2±58.1 | 272.3±88.6 | 439.0±68.4 | .002 | .006 | .000 |
| Total | 4325.6±449.0 | 3546.5±852.9 | 4377.7±568.8 | .008 | .751 | .025 |
CIEDs, cardiac implantable electronic devices; CRT-D, cardiac resynchronization therapy-defibrillators; CRT-P, cardiac resynchronization therapy-pacemakers; ICD, implantable cardiac defibrillators; ILR, implantable loop recorders; LLPM, leadless pacemakers; PM, pacemakers (single and dual chamber); S-ICD, subcutaneous implantable cardiac defibrillators.
The prelockdown period comprised January 2018 to February 2020; the lockdown period was from March to June 2020, and the postlockdown period was from July 20 to December 2021. The Student t test was used to compare the means. P <.05 was considered statistically significant.
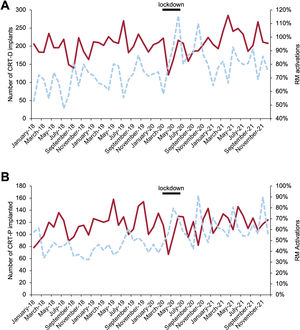
Central Illustration. Line chart: number of CIEDs implanted per month (left axis, top line) and percentage of CIEDs in which RM was activated, compared with devices implanted monthly (right axis, bottom line). The black line marks the lockdown period (March to June 2020). Bar chart: number of implants per year; table: percentage of RM activations, compared with devices implanted yearly. CIEDs, cardiac implantable electronic devices; CRT-D, cardiac resynchronization therapy-defibrillators; CRT-P, cardiac resynchronization therapy-pacemakers; ICD, implantable cardiac defibrillators; ILR, implantable loop recorders; LLPM, leadless pacemakers; PM, pacemakers (single and dual chamber); RM, remote monitoring; S-ICD, subcutaneous implantable cardiac defibrillators.
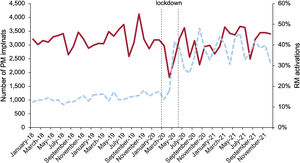
Pacemaker implantations (excluding CRT-P) decreased by 11.6% in 2020. This decrease was greater than the European average reduction of 6.6%.29 As shown in figure 2, there was a pronounced reduction during the lockdown, reaching 48% in April 2020 compared with April 2019, and a sharp upsurge when the lockdown ended, with maintenance of prepandemic implant rates thereafter. In 2021, there was a 12.8% increase in pacemaker implants compared with 2020, and a 3% increase compared with the average for 2018 to 2019.
Both the reduction in pacemaker implants in 2020 and the increase in 2021 were not homogeneous countrywide (figure 1 of the supplementary data). The number of implants in 2021 was similar to that in 2019, without compensating for the reduction in implants in 2020. As shown in table 1 and figure 3B, the reduction in implants did not affect leadless pacemakers (LLPM), which continued to increase in 2020 (13%). Considering all single and dual pacemakers plus LLPM based on the average for 2018 to 2019 average, 3905 pacemakers were not implanted in 2020. If we consider the increase in 2021, at least 7% of the average for 2018 to 2019 were not performed in Spain (table 1, table 2; figure 2; figure 1 of the supplementary data).
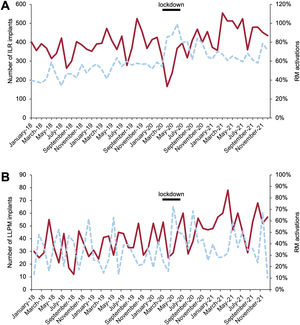
A: number of ILR implanted (solid line), percentage of RM activations (dashed line). B: number of LLPM implanted (solid line), and percentage of RM activations (dashed line). The black line marks the lockdown. ILR, implantable loop recorders; LLPM, leadless pacemakers; RM, remote monitoring.
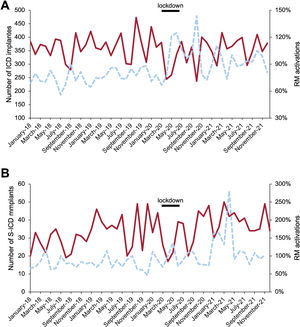
The reduction in ICD implantations in 2020 was 7% compared with 2019, which was similar to the European average reduction of 7.3% and 5.8% compared with the average for 2018 to 2019 (table 1, table 2; figure 4A, and figure 2 of the supplementary data).29 ICD implantations increased by 5.6% in 2021 compared with 2020 but did not increase compared with the average for 2018 to 2019, and therefore the loss of devices was not compensated. As shown in table 1 and figure 4B, S-ICD implantations continued to increase despite the pandemic, by 13% in 2020. However, this increase did not compensate for the reduction in the rest of ICD implants.
A: number of ICD implanted (solid line), percentage of RM activations (dashed line). B: number of S-ICD implanted (solid line), percentage of RM activations (dashed line). ICD, implantable cardiac defibrillators; RM, remote monitoring; S-ICD, subcutaneous implantable cardiac defibrillators.
Based on the average for 2018 to 2019, it is estimated that a total of 231 ICD+S-ICD were not implanted in 2020. Taking into account the increase in implants in 2021, at least 3% of the average for 2018 to 2019 were not performed in Spain, considering that this estimate did not reflect the upward trend in ICD implants observed in Spain in recent years.29,30
CRT implantationsThe pandemic led to a reduction in CRT implantations in 2020, which was subsequently compensated for. The total drop in the number of CRT-D implants in 2020 was 0.8% but increased by 15.4% in 2021 compared with 2020. The total balance for 2020 and 2021 represents an increase of 383 implants (16.5% of the average for 2018-2019) (table 1, table 2, figure 5A; figure 3 of the supplementary data). The reduction in CRT-P implants of 2.4% in 2020 and the 4.3% increase by 2021 represents an increase of 4.5%, taking the average for 2018 to 2019 as reference (table 1, table 2, figure 5B; figure 4 of the supplementary data).
A: number of CRT-D implanted (solid line), percentage of RM activations (dashed line). B: number of CRT-P implanted (solid line), percentage of RM activations (dashed line). CRT-D, cardiac resynchronization therapy-defibrillators; CRT-P, cardiac resynchronization therapy-pacemakers; RM, remote monitoring.
ILR implants decreased by 5.3% in 2020. The number of implantations significantly increased in 2021, reaching 21.6% compared with 2020 (table 1, table 2, figure 3A; figure 5 of the supplementary data).
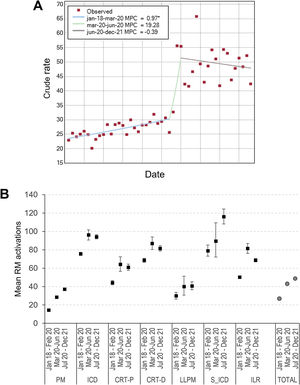
RM activationsThe percentage of RM activations of all CIEDs in Spain doubled from 24.5% in 2018 to 49.0% in 2021 (table 1, figure 1, figure 6 and figure 7).
A: regression analysis of the percentage of remote monitoring (RM) activations of all devices. The asterisk indicates that the monthly percent change (MPC) is different from zero at the alpha=0.05 level. B: mean and whisker plot showing percentage of RM activations. CIEDs, cardiac implantable electronic devices; CRT-D, cardiac resynchronization therapy-defibrillators; CRT-P, cardiac resynchronization therapy-pacemakers; ICD, implantable cardiac defibrillators; ILR, implantable loop recorders; LLPM, leadless pacemakers; PM, pacemakers (single and dual chamber); RM, remote monitoring; S-ICD, subcutaneous implantable cardiac defibrillators.
There was a marked change in trend from March to June 2020 (figure 7A). The rates of RM activations of CIEDs in the 3 predefined periods were significantly different (table 3).
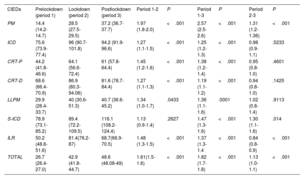
Comparison of means of the percentage of RM activations per implant of the different devices before, during and after COVID-19 pandemic lockdown
| CIEDs | Prelockdown (period 1) | Lockdown (period 2) | Postlockdown (period 3) | Period 1-2 | P | Period 1-3 | P | Period 2-3 | P |
|---|---|---|---|---|---|---|---|---|---|
| PM | 14.4 (14.2-14.7) | 28.5 (27.5-29.5) | 37.2 (36.7-37.7) | 1.97 (1.8-2.0) | <.001 | 2.57 (2.5-2.6) | <.001 | 1.31 (1.2- 1.36) | <.001 |
| ICD | 75.6 (73.9-77.4) | 96 (90.7-101.8) | 94.2 (91.9-96.6) | 1.27 (1.1-1.5) | <.001 | 1.25 (1.2-1.3) | <.001 | 0.98 (0.9-1.1) | .5233 |
| CRT-P | 44.2 (41.8-46.6) | 64.1 (56.6-72.4) | 61 (57.8-64.4) | 1.45 (1.2-1.6) | <.001 | 1.38 (1.2-1.4) | <.001 | 0.95 (0.8-1.0) | .4601 |
| CRT-D | 68.6 (66.4-70.9) | 86.9 (80.3-94.06) | 81.6 (78.7-84.4) | 1.27 (1.1-1.3) | <.001 | 1.19 (1.1-1.2) | <.001 | 0.94 (0.8-1.0) | .1425 |
| LLPM | 29.9 (26.4-33.7) | 40 (30.6-51.3) | 40.7 (36.6-45.2) | 1.34 (1.0-1.7) | .0433 | 1.36 (1.1-1.6) | .0001 | 1.02 (0.8-1.4) | .9113 |
| S-ICD | 78.9 (73.1-85.2) | 89.4 (72.2-109.5) | 116.1 (108.2-124.4) | 1.13 (0.9-1.4) | .2627 | 1.47 (1.3-1.6) | <.001 | 1.30 (1.1-1.6) | .014 |
| ILR | 50.2 (48.8-51.6) | 81.4(76.2-87) | 68.7(66.9-70.5) | 1.48 (1.3-1.5) | <.001 | 1.37 (1.3-1.4 | <.001 | 0.84 (0.8-0.9) | <.001 |
| TOTAL | 26.7 (26.4-27.0) | 42.9 (41.8-44.7) | 48.6 (48.08-49) | 1.61(1.5-1.6) | <.001 | 1.82 (1.7-1.8) | <.001 | 1.13 (1.0-1.1) | <.001 |
CIEDs, cardiac implantable electronic devices; CRT-D, cardiac resynchronization therapy-defibrillators; CRT-P, cardiac resynchronization therapy-pacemakers; ICD, implantable cardiac defibrillators; ILR, implantable loop recorders; LLPM, leadless pacemakers; PM, pacemakers (single and dual chamber); S-ICD, subcutaneous implantable cardiac defibrillators.
Data represent mean of the percentage of RM activations/implants and 95% confidence interval. The prelockdown period was from January 2018 to February 2020, the lockdown period was from March to June 2020, and the postlockdown was from July 20 to December 2021.
The rate of pacemaker RM activations was 14.3% in 2018, rising to 39.4% in 2021. The rates of RM activations for single or dual chamber pacemakers increased from 13.2% in 2018 to 38.6% in 2021. Of note, in 2020 the percentage of RM activations doubled (figure 2 and figures 6 and 11 of the supplementary data).
The rates of RM activations/implants (grouped in 3 periods) differed significantly (table 3). Single and dual chamber pacemakers were the only low-energy devices that continued to increase the percentage of RM activations in the 3 periods.
The progression in LLPM activation rates increased from 31.8% in 2018 to 38.6% in 2021 (table 1, table 3, figure 3B, and figure 7).
ICD RM activationsThe RM activation rates for ICD were 70.6% in 2018, rising to 90.1% in 2021 (table 1, table 3, figure 4A, figure 7; and figures 7 and 12 of the supplementary data). The activation rate was 72.9% in 2018 and 93% in 2021 (figure 4A).
The rates of RM activations/implants were statistically significant only on comparison of the first with the second and third periods (table 3). The activation rate for S-ICD was high at the beginning (78.7%) and became higher in 2021, including previously implanted devices (130%) (table 1, table 3, figure 4B).
CRT RM activationsRM activation rates of CRT-D in 2018 were 65.2% and rose to 78.7% in 2021 (table 1, table 3, figure 5A, figure 7; and figures 8 and 13 of the supplementary data). The differences in the rate of activations were statistically significant only when on comparison of the first with the second and third periods (table 3). CRT-P activation rates increased from 42.6% in 2018 to 63.1% in 2021 (table 1, table 3, figure 5B; and figure 14 of the supplementary data).
ILR RM activationsThe percentage of ILR activated for RM increased from 44% to 65% (2018-2021) (table 1). When the 3 periods are compared, there was a large increase in RM during the lockdown (81.4%). Subsequently there was a significant reduction (68.7%), but values were still well above prelockdown values (50.2%) (table 1, table 3, figure 3A; and figures 10 and 15 of the supplementary data).
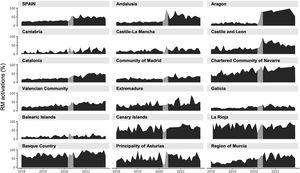
Factors influencing RM activationsWhen the different regions of Spain were analyzed, large differences were observed in RM activations (figures 6-10 of the supplementary data). Regions with a higher implantation rate per inhabitant did not have a higher percentage of RM activations (table 1 of the supplementary data). We found no correlation between the percentage of RM activations in the different regions with the impact of the pandemic on the health system (figures 16 and 17 of the supplementary data), or with the economic status of each community (calculated using the gross domestic product per capita) (table 2 of the supplementary data). We also analyzed whether the device manufacturer was a relevant factor in terms of activations, but this was only true for single and dual chamber pacemakers and ICDs, but the regions were acting as an iteration variable, so the distribution of implants and RM activations per manufacturer were completely different in each region (figures 18 and 19AB of the supplementary data). For all other devices, most manufacturers had very similar figures (figures 20-22AB of the supplementary data).
DISCUSSIONThe COVID-19 RM Spain Registry collected national data on implantations and RM activations of CIEDs. Considering the methodology, and the acceptance of all the institutions to share their data, we estimate that we recorded all the data on implants and RM activations of CIEDs in Spain from 2018 to 2021. We observed a sharp drop in implants during lockdown with a recovery for most devices in 2021, except for pacemakers and ICDs. RM increased dramatically for all devices during the lockdown and has subsequently remained at higher levels.
Spain consists of 17 regions (autonomous communities) and 2 autonomous cities. The national health system provides universal health care to all citizens and the administration of the system is devolved to the autonomous communities. Most of the institutions conducting implants are public. We found large differences in the percentage of implants per inhabitant among regions, with no clear reasons for these differences, as previously reported.30
All regions showed a fairly homogeneous reduction in implants in 2020, but patterns differed in 2021 depending on the type of device and region.
Prior studies have demonstrated a significant reduction in CIED implantations during the lockdown period, with 24% to 35% in urgent pacemakers,20,21 44% to 55% in all pacemakers, 45% to 64% in defibrillators, 42% in CRT-P and 46% in CRT-D.19,22 In our registry, we were able to verify that following this reduction, there was a slow recovery, which in many cases reached the same number of implants as in the prepandemic period. However, it appears that a significant number of implants were not postponed, but “lost”. This trend was most evident in devices with a higher number of implants, and was very marked in pacemakers and less evident in ICDs, whereas CRT, ILR and newer devices such as S-ICD and LLPM do not seem to have been affected.
The reasons for the reduction in implants throughout 2020 may be multifactorial: postponement of all nonurgent interventions, saturation of health care resources, fear of contagion, and even competing risk due to higher mortality.
In addition, the drop in pacemaker implantations in Spain in 2020 almost doubled the European average with no clear explanation. A national registry was unable to demonstrate a causal relationship between the reduction in urgent pacemaker implantations and higher mortality and hospital pressure during lockdown.20
European trends in recent years, following the publication of the DANISH trial,29,32 have shown a stagnation in ICD implantations and a reduction in CRT-D (with an increase in CRT-P). In Spain, however, and probably because the implant rates were well below the European average, the prepandemic trend showed a progressive increase in these 3 types of devices and consequently the loss of implants could probably be even greater than previously mentioned. Considering the previous trend from 2018 to 2019 and the overall number of implants in 2020 and 2021, we found a significant overall increase in CRT-D an CRT-P and a reduction in single and dual chamber ICDs. This pattern is repeated in the European trend for implants reported by Medtech.29
A possible explanation for the reduction in ICD implants is that the pandemic led to a decrease in indications for ICD as primary prevention due to the drop in in-office evaluations, and as secondary prevention due to an increase in sudden death, reduction in resuscitation attempts,15,16 as well as a decrease in ventricular arrhythmias due to limited activities.33
The upgrade rates from ICD to CRT-D range from 5% to 11%34,35 in treatment-optimized patients during the lifetime of the device, but the reduction in in-person evaluations made diagnosis and optimized treatment impossible, which may have led to earlier and greater clinical deterioration. All of the above could explain the fact that ICD implantations were reduced and that even the initial indications for primary prevention finally became CRT-D/CRT-P indications.
The percentage of RM activations increased significantly. There was a marked rise in activations coinciding with the lockdown and a second increase in the fall of 2020 at the time of the second wave and probably with the lessons learned from the first wave. Since lockdown, pacemakers and S-ICDs have continued to increase, whereas activations of the remaining devices have been reduced, despite maintaining activation percentages well above the prepandemic figures.
Our results differ from those of the EHRA survey,27 which found significant differences only in pacemakers and ILR RM activations. The differences are probably due to the inclusion of different countries, the selection bias of the survey due to voluntary date reporting, and the sum of percentages performed by the EHRA survey instead of the sum of absolute numbers.
The activation of RM increased in most regions, but its distribution is widely heterogeneous, being high in some regions and almost nonexistent in others. For pacemakers, the variability in the percentage of RM activations between regions was substantial. These effects were not influenced by the impact of the pandemic on the health system, regional wealth, the number of implants, or even the geographical dispersion.
For high-voltage devices, the initial figures for RM were high and increased in 2021 to 93% for ICD and above 100% for S-ICD devices.
It is worth highlighting the CRT-D group which, although its percentage increased, was below the activation figures of the other ICDs, reaching 78% in 2021. There is evidence to suggest that the greatest clinical benefit of RM can be found in the patients with poorer health (which would include most CRT-D patients).9,36 However, the need to perform ECGs to validate the correct functioning of the device makes RM less efficient than other high-voltage devices in terms of reducing in-office evaluations.
According to a substudy of the US ALTITUDE registry, the factors influencing RM activations are socioeconomic but the determining factor is the institution/physicians.37 In our study, RM activations were determined by the decision of the centers to implement it. In this regard, the COVID-19 pandemic seemed to have convinced many physicians and institutions to activate RM.
The main limitation for the implementation of RM is the lack of reimbursement.13,14,27 In Spain there is currently no specific reimbursement for RM for the manufacturers or for physicians/institutions.13,38
To perform quality RM, it is necessary to provide units with human and material resources, especially multiplatform software or external centralized monitoring systems that allow the management and integration of the information obtained from all the manufacturers’ RM systems with the electronic health records of the institutions. The increase in the number of CIEDs with activated RM has not been due to major changes in health policies, but rather to staff reorganization and the efforts of manufacturers in response to the pandemic. However, this limitation of resources may hinder the future sustainability of this technology.13 As evidence, we can observe that after the lockdown, the upward trend in RM activations has not been sustained in all devices. To the best of our knowledge, this is the first study in which information from a whole country on implantations and RM activations has been obtained directly from all the manufacturers of CIEDs who maintain and own the servers supporting RM. Therefore, they are the most reliable source of information on the total number of RM activations performed in their systems.
The anonymization of the data, using the overall sum of both implants and RM activations for all manufacturers and all the centers nationwide, has allowed us to compile a highly comprehensive collection of all data and thus eliminate the biases that inevitably arise from voluntary surveys collecting the percentage of activations.
LimitationsThe data collection did not provide us with information on the number of primo-implants/replacements, age, sex or socioeconomic status.
RM activation was according to each institution's preferences, and therefore there may be some inter-monthly variations that were finally compensated for in the overall annual average. The study design addressed the RM activation rate but not the percentage of time under follow-up, nor did it identify patients who had RM or only performed scheduled remote interrogations. Finally, the implementation of RM may have been limited because it may not have been accepted in the current tenders for CIEDs in some public institutions.
CONCLUSIONSIn 2020 there was a sharp reduction in implantations of all CIEDs, which gradually returned to prepandemic figures. In the most common devices (pacemakers and ICDs), unlike the other devices, the reduction in 2020 was not compensated for by the increment in 2021. The prepandemic trend in CRT-D reduction changed, with implants increasing highly significantly by 2021. The pandemic seems to have changed how implantable cardiac devices are monitored in Spain, representing an unprecedented upheaval for RM. However, the ideal conditions have not yet been met in terms of recognition of this overload of work or the provision of the necessary resources by the health authorities.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSF.J. García-Fernández: conceptualization, lead; data curation, lead; formal analysis, lead; methodology, lead; writing–original draft, lead; writing–review and editing, lead. S Calvo Simal: data curation, equal; formal analysis: lead; methodology: lead; writing–review and editing: supporting. Ó Cano Pérez: project administration: supporting; resources: equal; supervision: supporting; writing–review and editing: supporting). D. Calvo Cuervo: supervision: supporting; writing–review and editing: supporting. M. Pombo Jiménez: supervision: supporting; writing–review and editing: supporting. I. Fernández Lozano: supervision: supporting; writing–review and editing: supporting. L. Villagraz Tercedor: supervision: supporting; validation: supporting; writing–review and editing: supporting). G. Fernández Palacios: writing–review and editing: supporting. J. Martín González: supervision: supporting; validation: supporting; writing–review and editing: supporting.
CONFLICTS OF INTERESTF. J. García-Fernández received a research grant from Biotronik, Speaker's fees from Medtronic, Boston Sci; D. Calvo Cuervo is President of the Spanish Heart Rhythm Association; Ó Cano Pérez received consulting and lecturer's fees from Abbott, Biotronik, Boston Sci, and Medtronic; I. Fernández Lozano is the recipient of institutional grants from the Spanish Society of Cardiology and the Fundación para Investigación Cardiovascular and received advisor/consulting and speaker personal fees from Bayer, Medtronic and Abbott; all the other authors have no conflicts of interest to disclose.
The authors would like to thank the Information and Statistics Area of the Spanish Ministry of Health, Social Services and Equality for providing them with current data on the hospital burden of the pandemic and the manufacturers for their generosity and extraordinary accuracy in providing them with information, especially Carlos Briz (Boston Scientific), Luis García (Microport), Araceli Montero (Medtronic), Marina Rujas (Biotronik), and and Estela Silvan (Abbott) for their outstanding efforts. The authors thank Francisco López de Saro (Trialance SCCL) for editorial support.