Patients with a current acute coronary syndrome and previous ischemic heart disease, peripheral arterial disease, and/or cerebrovascular disease are reported to have a poorer outcome than those without these previous conditions. It is uncertain whether this association with outcome is observed at long-term follow-up.
MethodsProspective observational study, including 4247 patients with ST-segment elevation myocardial infarction. Detailed clinical data and information on previous ischemic heart disease, peripheral arterial disease, and cerebrovascular disease («vascular burden») were recorded. Multivariate models were performed for in-hospital and long-term (median, 7.2 years) all-cause mortality.
ResultsOne vascular territory was affected in 1131 (26.6%) patients and ≥ 2 territories in 221 (5.2%). The total in-hospital mortality rate was 12.3% and the long-term incidence density was 3.5 deaths per 100 patient-years. A background of previous ischemic heart disease (odds ratio = 0.83; P = .35), peripheral arterial disease (odds ratio = 1.30; P = .34), or cerebrovascular disease (stroke) (odds ratio = 1.15; P = .59) was not independently predictive of in-hospital death. In an adjusted model, previous cerebrovascular disease and previous peripheral arterial disease were both predictors of mortality at long-term follow-up (hazard ratio = 1.57; P < .001; and hazard ratio = 1.34; P = .001; respectively). Patients with ≥ 2 diseased vascular territories showed higher long-term mortality (hazard ratio = 2.35; P < .001), but not higher in-hospital mortality (odds ratio = 1.07; P = .844).
ConclusionsIn patients with a diagnosis of ST-segment elevation acute myocardial infarction, the previous vascular burden determines greater long-term mortality. Considered individually, previous cerebrovascular disease and peripheral arterial disease were predictors of mortality at long-term after hospital discharge.
Keywords
Atherosclerosis is a chronic, progressive systemic arterial disease that can affect the coronary arteries as acute myocardial infarction or angina, the arteries of the lower extremities as peripheral arterial disease (PAD), and the cerebral vasculature as cerebrovascular disease (CVD), mainly stroke.1 Thus, the concept of polyvascular disease has emerged to refer to patients with > 1 affected vascular territory (the so-called “previous vascular burden”) because of the existing etiologic nexus.2 The importance of polyvascular disease resides in reported evidence that atherosclerotic involvement of ≥ 1 vascular territories leads to underuse of medications with proven benefits3–8 and fewer coronary revascularization treatments,3,4,7,9,10 which has an adverse impact on the clinical course during hospitalization3,6–9,11 and at follow-up.4,7,8,11–13 These observations have been investigated for each of the 3 territories separately,4,6,7,10–12 and for concomitant involvement of 2 territories, mainly CVD and PAD.8 However, few studies have assessed the impact of previous involvement of all 3 territories mentioned—ischemic heart disease (IHD), PAD, and/or CVD—on in-hospital management and mortality,3,9 and, to our knowledge, there is no published information on the long-term status following hospital discharge.
A recent study in Spain8 investigated the adverse prognostic value of previous PAD and/or CVD for in-hospital and 6-month mortality in a heterogeneous population of patients with acute coronary syndrome (ACS). The population ranged from patients with a very low risk status, diagnosed by stress testing or simply by their history of IHD, to those at high or very high risk (high-risk ST-segment elevation or non–ST-segment elevation ACS). Nonetheless, it is unknown whether the prognostic performance of a history of PAD and/or CVD is similar in the different types of ACS according to the risk stratum. The long-term (> 1 year) impact is also uncertain.
The aim of this study is to evaluate the prognostic importance of the previous vascular burden during hospitalization and at long-term following discharge in hospitalized patients with a diagnosis of persistent ST-segment elevation acute myocardial infarction (STEAMI).
METHODSEnrollmentBetween January 1998 and January 2008, we enrolled all patients consecutively hospitalized with a diagnosis of STEAMI in the coronary units of 2 university hospitals in the Region of Murcia: Hospital Universitario Virgen de la Arrixaca (El Palmar) and Hospital Universitario de Santa Lucía (Cartagena). The patients were included in a prospective, longitudinal, observational study. Patients who experienced an acute myocardial infarction during a coronary revascularization procedure were excluded.
Diagnosis of STEAMI was based on the presence of typical chest pain of ≥ 30 min duration and/or elevated myocardial necrosis markers, together with a presumably new ST segment elevation in ≥ 2 precordial leads: > 0.2 mm in V1, V2 or V3, and > 0.1 mm in the lateral (aVL, I) or inferior (II, III, and aVF) leads. Patients with a presumably new left bundle branch block were also included.
The study was approved by the ethics committee of each participating center, and patients gave written consent to be included in the registry.
Variables. DefinitionsComplete demographic information was collected for each patient. Previous IHD was established from documented previous diagnosis of angina or myocardial infarction, or on surgical or percutaneous coronary revascularization. Previous PAD was defined as a documented history of peripheral arterial disease, claudication, amputation due to arterial failure, aortoiliac occlusive disease, surgical or percutaneous peripheral arterial revascularization, or positive results on noninvasive testing. Previous CVD (stroke) was established with a documented history of sudden-onset loss of neurologic function that persisted in time. The previous vascular burden was defined as the “number” of diseased vascular territories, basically previous IHD, CVD, or PAD. Severe or major bleeding complications were defined as cerebral and retroperitoneal hemorrhage, and bleeding at any other site resulting in hemodynamic deterioration and/or the need for transfusion of whole blood or blood products.
After hospital discharge, patients underwent lengthy follow-up (median, 7.2 years) by telephone contact, medical record review, outpatient visits, and review of death registries. In-hospital deaths were excluded from this analysis. Follow-up information was obtained for 98% of the study participants.
Statistical AnalysisContingency tables and the chi-square test or Fisher exact test, as appropriate, were used to determine relationships between dichotomous variables. Qualitative variables were compared using analysis of variance or the Kruskal-Wallis test, as appropriate. Factors associated with in-hospital death were analyzed by binary multivariate regression analysis. Odds ratios (ORs) were calculated with their respective 95% confidence intervals (95%CIs). The survival analysis following discharge was performed using Kaplan-Meier graphs, and between-group comparisons were done with the Mantel-Haenszel test. Cox regression was carried out, estimating the hazard ratio (HR) and 95%CI as a measure of associations. Variables with a non-Gaussian distribution were transformed by log10. The following variables were chosen as confounders for the multivariate models: a) variables in the study that showed an association with either the variable of interest (all-cause death) or the number of affected vascular territories, with a P value < .05, and b) variables that showed an association consistent with the variable of interest in previous studies. In addition, we included variables that achieved a crude OR/HR > 10% for the variable “territories”. Covariates were entered in blocks, the variables of interest (number of diseased vascular territories, IHD, PAD, previous CVD) using the “enter” option and the confounders using a backward stepwise method, applying the Wald statistic. The log linear assumption was checked by constructing graphs. The discrimination of the final model was estimated using the C statistic, and the calibration using the Hosmer-Lemeshow test. The proportional hazards assumption was checked using the Schoenfeld residuals test and graphs. Harrell C statistic was calculated for the Cox regression models. The 95%Cls for the survival model were additionally calculated by resampling (bootstrapping) with 3000 iterations. The percentage of missing values per variable was, in general, < 2% for most variables (99%). A P value of < .05 was considered statistically significant. All analyses were performed with the PASW statistical package, version 20 (IBM, United States) and STATA 9.1 (College Station, Texas, United States).
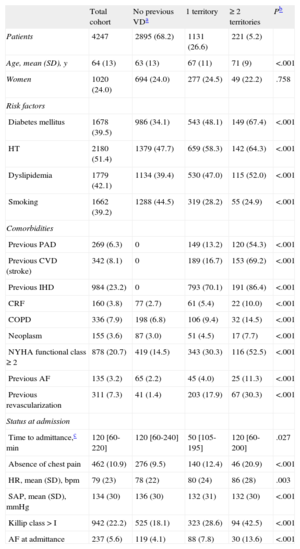
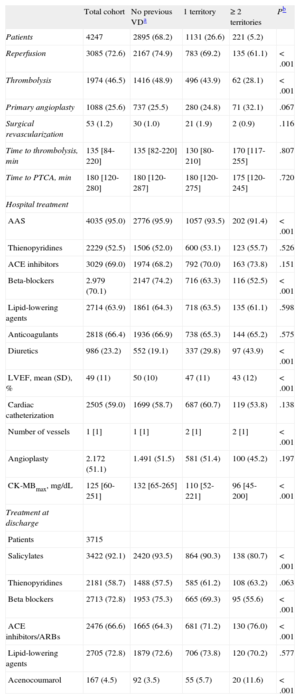
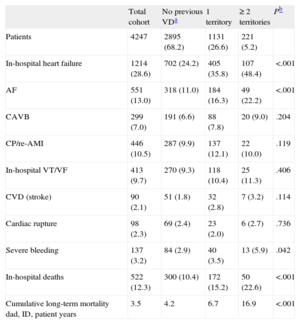
RESULTSBaseline Characteristics of the SampleThe baseline characteristics of the study sample (n = 4247) are shown in Table 1. Mean age was 64 years and 24.0% were women. Previous vascular involvement included IHD in 23.2%, PAD in 6.3%, and CVD in 8.1%. The number of vascular territories affected was 1 territory in 26.6%, 2 territories in 4.7%, and all 3 territories in only 0.5%. Because there were only 22 patients with 3 affected territories, we created the category “≥ 2 diseased territories” for the statistical analyses. The in-hospital treatment, including reperfusion therapy, and treatment after hospital discharge are shown in Table 2. In-hospital complications are presented in Table 3.
Baseline Characteristics. Medical History and Clinical Status at Admission
| Total cohort | No previous VDa | 1 territory | ≥ 2 territories | Pb | |
| Patients | 4247 | 2895 (68.2) | 1131 (26.6) | 221 (5.2) | |
| Age, mean (SD), y | 64 (13) | 63 (13) | 67 (11) | 71 (9) | <.001 |
| Women | 1020 (24.0) | 694 (24.0) | 277 (24.5) | 49 (22.2) | .758 |
| Risk factors | |||||
| Diabetes mellitus | 1678 (39.5) | 986 (34.1) | 543 (48.1) | 149 (67.4) | <.001 |
| HT | 2180 (51.4) | 1379 (47.7) | 659 (58.3) | 142 (64.3) | <.001 |
| Dyslipidemia | 1779 (42.1) | 1134 (39.4) | 530 (47.0) | 115 (52.0) | <.001 |
| Smoking | 1662 (39.2) | 1288 (44.5) | 319 (28.2) | 55 (24.9) | <.001 |
| Comorbidities | |||||
| Previous PAD | 269 (6.3) | 0 | 149 (13.2) | 120 (54.3) | <.001 |
| Previous CVD (stroke) | 342 (8.1) | 0 | 189 (16.7) | 153 (69.2) | <.001 |
| Previous IHD | 984 (23.2) | 0 | 793 (70.1) | 191 (86.4) | <.001 |
| CRF | 160 (3.8) | 77 (2.7) | 61 (5.4) | 22 (10.0) | <.001 |
| COPD | 336 (7.9) | 198 (6.8) | 106 (9.4) | 32 (14.5) | <.001 |
| Neoplasm | 155 (3.6) | 87 (3.0) | 51 (4.5) | 17 (7.7) | <.001 |
| NYHA functional class ≥ 2 | 878 (20.7) | 419 (14.5) | 343 (30.3) | 116 (52.5) | <.001 |
| Previous AF | 135 (3.2) | 65 (2.2) | 45 (4.0) | 25 (11.3) | <.001 |
| Previous revascularization | 311 (7.3) | 41 (1.4) | 203 (17.9) | 67 (30.3) | <.001 |
| Status at admission | |||||
| Time to admittance,c min | 120 [60-220] | 120 [60-240] | 50 [105-195] | 120 [60-200] | .027 |
| Absence of chest pain | 462 (10.9) | 276 (9.5) | 140 (12.4) | 46 (20.9) | <.001 |
| HR, mean (SD), bpm | 79 (23) | 78 (22) | 80 (24) | 86 (28) | .003 |
| SAP, mean (SD), mmHg | 134 (30) | 136 (30) | 132 (31) | 132 (30) | <.001 |
| Killip class > I | 942 (22.2) | 525 (18.1) | 323 (28.6) | 94 (42.5) | <.001 |
| AF at admittance | 237 (5.6) | 119 (4.1) | 88 (7.8) | 30 (13.6) | <.001 |
AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease, CRF, chronic renal failure; CVD, cerebrovascular disease; IHD, ischemic heart disease; HR, heart rate; HT, hypertension; NYHA, New York Heart Association; PAD, peripheral arterial disease; SAP, systolic arterial pressure; SD, standard deviation; VD, vascular disease.
Unless otherwise indicated, the data are expressed as No. (%) or median [interquartile range].
Reperfusion and Treatment During Hospitalization and at Discharge
| Total cohort | No previous VDa | 1 territory | ≥ 2 territories | Pb | |
| Patients | 4247 | 2895 (68.2) | 1131 (26.6) | 221 (5.2) | |
| Reperfusion | 3085 (72.6) | 2167 (74.9) | 783 (69.2) | 135 (61.1) | <.001 |
| Thrombolysis | 1974 (46.5) | 1416 (48.9) | 496 (43.9) | 62 (28.1) | < .001 |
| Primary angioplasty | 1088 (25.6) | 737 (25.5) | 280 (24.8) | 71 (32.1) | .067 |
| Surgical revascularization | 53 (1.2) | 30 (1.0) | 21 (1.9) | 2 (0.9) | .116 |
| Time to thrombolysis, min | 135 [84-220] | 135 [82-220] | 130 [80-210] | 170 [117-255] | .807 |
| Time to PTCA, min | 180 [120-280] | 180 [120-287] | 180 [120-275] | 175 [120-245] | .720 |
| Hospital treatment | |||||
| AAS | 4035 (95.0) | 2776 (95.9) | 1057 (93.5) | 202 (91.4) | <.001 |
| Thienopyridines | 2229 (52.5) | 1506 (52.0) | 600 (53.1) | 123 (55.7) | .526 |
| ACE inhibitors | 3029 (69.0) | 1974 (68.2) | 792 (70.0) | 163 (73.8) | .151 |
| Beta-blockers | 2.979 (70.1) | 2147 (74.2) | 716 (63.3) | 116 (52.5) | <.001 |
| Lipid-lowering agents | 2714 (63.9) | 1861 (64.3) | 718 (63.5) | 135 (61.1) | .598 |
| Anticoagulants | 2818 (66.4) | 1936 (66.9) | 738 (65.3) | 144 (65.2) | .575 |
| Diuretics | 986 (23.2) | 552 (19.1) | 337 (29.8) | 97 (43.9) | <.001 |
| LVEF, mean (SD), % | 49 (11) | 50 (10) | 47 (11) | 43 (12) | <.001 |
| Cardiac catheterization | 2505 (59.0) | 1699 (58.7) | 687 (60.7) | 119 (53.8) | .138 |
| Number of vessels | 1 [1] | 1 [1] | 2 [1] | 2 [1] | <.001 |
| Angioplasty | 2.172 (51.1) | 1.491 (51.5) | 581 (51.4) | 100 (45.2) | .197 |
| CK-MBmax, mg/dL | 125 [60-251] | 132 [65-265] | 110 [52-221] | 96 [45-200] | <.001 |
| Treatment at discharge | |||||
| Patients | 3715 | ||||
| Salicylates | 3422 (92.1) | 2420 (93.5) | 864 (90.3) | 138 (80.7) | <.001 |
| Thienopyridines | 2181 (58.7) | 1488 (57.5) | 585 (61.2) | 108 (63.2) | .063 |
| Beta blockers | 2713 (72.8) | 1953 (75.3) | 665 (69.3) | 95 (55.6) | <.001 |
| ACE inhibitors/ARBs | 2476 (66.6) | 1665 (64.3) | 681 (71.2) | 130 (76.0) | <.001 |
| Lipid-lowering agents | 2705 (72.8) | 1879 (72.6) | 706 (73.8) | 120 (70.2) | .577 |
| Acenocoumarol | 167 (4.5) | 92 (3.5) | 55 (5.7) | 20 (11.6) | <.001 |
AAS, acetylsalicylic acid; ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; CK-MB, creatine-kinase MB isoenzyme; LVEF, left ventricular ejection; PTCA, percutaneous transluminal coronary angioplasty; SD, standard deviation; VD: vascular disease.
The percentage is calculated over the total of fibrinolysis. “Time to thrombolysis” is defined as the time between the onset of chest pain or the first guiding symptom and the start of systemic thrombolysis. “Time to percutaneous transluminal coronary angioplasty” is defined as the time between the onset of chest pain or the first guiding symptom and the start of coronary angioplasty.
Unless otherwise indicated, the data are expressed as No. (%) or median [interquartile range].
In-hospital Complications and Deaths. Mortality During Hospitalization and at Long-term
| Total cohort | No previous VDa | 1 territory | ≥2 territories | Pb | |
| Patients | 4247 | 2895 (68.2) | 1131 (26.6) | 221 (5.2) | |
| In-hospital heart failure | 1214 (28.6) | 702 (24.2) | 405 (35.8) | 107 (48.4) | <.001 |
| AF | 551 (13.0) | 318 (11.0) | 184 (16.3) | 49 (22.2) | <.001 |
| CAVB | 299 (7.0) | 191 (6.6) | 88 (7.8) | 20 (9.0) | .204 |
| CP/re-AMI | 446 (10.5) | 287 (9.9) | 137 (12.1) | 22 (10.0) | .119 |
| In-hospital VT/VF | 413 (9.7) | 270 (9.3) | 118 (10.4) | 25 (11.3) | .406 |
| CVD (stroke) | 90 (2.1) | 51 (1.8) | 32 (2.8) | 7 (3.2) | .114 |
| Cardiac rupture | 98 (2.3) | 69 (2.4) | 23 (2.0) | 6 (2.7) | .736 |
| Severe bleeding | 137 (3.2) | 84 (2.9) | 40 (3.5) | 13 (5.9) | .042 |
| In-hospital deaths | 522 (12.3) | 300 (10.4) | 172 (15.2) | 50 (22.6) | <.001 |
| Cumulative long-term mortality dad, ID, patient years | 3.5 | 4.2 | 6.7 | 16.9 | <.001 |
AF, atrial fibrillation; AMI, acute myocardial infarction; CAVB, complete atrioventricular block; CP, chest pain; CVD, cerebrovascular disease; ID, incidence density; VD, vascular disease; VF, ventricular fibrillation; VT, ventricular tachycardia.
Unless otherwise indicated, values are expressed as No. (%).
Patients with a larger number of diseased territories were older and had greater exposure to classic cardiovascular risk factors, more comorbid conditions, and previous atrial fibrillation (Table 1). Regarding the clinical presentation at hospitalization, patients with a greater previous vascular burden were more likely to have signs of heart failure and no complaints of chest pain. In general, these patients underwent reperfusion less often than the remaining cohort. At discharge, patients with a greater previous vascular burden were less commonly prescribed salicylates and beta blockers, but more often received anticoagulants and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.
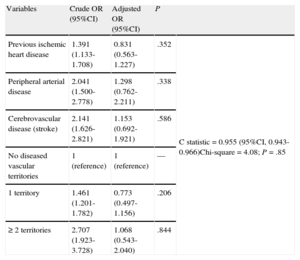
Complications and In-hospital Mortality According to Vascular BurdenPatients with a greater vascular burden were more likely to have heart failure, atrial fibrillation, and major bleeding during hospitalization. In-hospital mortality progressively increased in parallel to the number of diseased territories, from 10.4% in patients with no previous vascular disease to 22.6% in those with ≥ 2 diseased territories (P for trend < .001). Taken individually, in-hospital mortality was 15.1% in patients with previous IHD, 21.2% in those with previous PAD, and 21.6% in those with previous CVD. There were 522 deaths by any cause (12.3%). The binary multivariate logistic regression model for predicting in-hospital death is shown in Table 4. Previous IHD (OR = 0.83; 95%CI 0.56-1.23), previous PAD (OR = 1.30; 95%CI 0.76-2.21), and previous CVD (OR = 1.15; 95% CI, 0.69-1.92) were not predictive of in-hospital mortality. The independent risk factors for death in this model were age, female sex, heart rate and systolic arterial pressure at admittance, Killip class > 1, diabetes mellitus, previous atrial fibrillation, previous New York Heart Association functional class ≥ 2, previous chronic renal failure, in-hospital angina or reinfarction, development of complete atrioventricular block, in-hospital tachycardia/ventricular fibrillation, cardiac rupture, and major bleeding. The variable “number of vascular territories” was also found to lack predictive value for in-hospital mortality. In relation to these findings, the supplementary material in Table 1S shows the associations between vascular burden and in-hospital death in models adjusted incrementally, and Table 2S depicts the complete multivariate model.
Crude and Adjusted Binary Logistic Regression Models for In-hospital Mortality
| Variables | Crude OR (95%CI) | Adjusted OR (95%CI) | P | |
| Previous ischemic heart disease | 1.391 (1.133-1.708) | 0.831 (0.563-1.227) | .352 | C statistic = 0.955 (95%CI, 0.943-0.966)Chi-square = 4.08; P = .85 |
| Peripheral arterial disease | 2.041 (1.500-2.778) | 1.298 (0.762-2.211) | .338 | |
| Cerebrovascular disease (stroke) | 2.141 (1.626-2.821) | 1.153 (0.692-1.921) | .586 | |
| No diseased vascular territories | 1 (reference) | 1 (reference) | — | |
| 1 territory | 1.461 (1.201-1.782) | 0.773 (0.497-1.156) | .206 | |
| ≥2 territories | 2.707 (1.923-3.728) | 1.068 (0.543-2.040) | .844 |
OR, odds ratio; 95%CI, 95% confidence interval.
Adjusted by age, heart rate, blood pressure, sex, hypercholesterolemia, diabetes mellitus, previous atrial fibrillation, baseline New York Heart Association functional class ≥2, previous renal failure, Killip class at admittance, reinfarction, complete atrioventricular block, in-hospital tachycardia or ventricular fibrillation, cardiac rupture, major bleeding, creatine-kinase MB isoenzyme peak, left ventricular ejection fraction, and treatment with acetylsalicylic acid, beta blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and lipid-lowering agents.
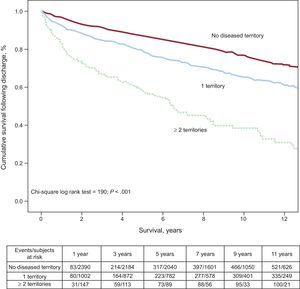
In the present study, follow-up lasted a median of 7.2 years [interquartile range, 2.7-10.3 years]. A total of 1023 deaths were recorded after discharge, yielding an incidence density for long-term mortality of 3.5/100 patient-years. The incidence density of post-hospitalization mortality was 4.2/100 patient-years in patients with no previous vascular involvement, 6.7/100 patient-years in those with 1 diseased territory, and 16.9/100 patient-years in those with ≥ 2 affected territories (P for trend < .001). By individual territory, mortality was 34.1% in patients with previous IHD vs 24.6% (P < .001) in patients without this background, 52.1% in patients with previous PAD vs 25.6% in those without (P < .001), and 46.4% in those with previous CVD vs 25.6% in those without (P < .001). The long-term survival curve according to the number of diseased vascular territories is depicted in Figure 1. Patients who had died within 1 year (data not shown) were older and a larger percentage were women, diabetic, and hypertensive, and they were less often smokers. As to the vascular burden, these patients had a significantly more frequent personal history of previous IHD, PAD, or CVD. In addition, they had greater comorbidity, less chest pain upon arrival to the hospital, a higher heart rate, and more frequent heart failure at admittance. Patients who died had undergone reperfusion less often and had more in-hospital complications, particularly heart failure. Of note, at hospital discharge, these patients received salicylates, beta blockers and lipid-lowering agents less often, but were more frequently prescribed angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and acenocoumarol.
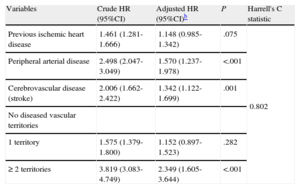
In the multivariate analysis (Table 5), the previous vascular burden and a history of PAD or CVD were both independent predictors of all-cause death. Previous IHD showed a trend approaching statistical significance (HR = 1.148; P = .075) (Table 5). In the supplementary material, Table 3S depicts the associations between vascular burden and long-term mortality in incrementally adjusted models, and Table 4S shows the complete multivariate model.
Crude and Adjusted Cox Regression Models for Long-term Mortality Following Dischargea
| Variables | Crude HR (95%CI) | Adjusted HR (95%CI)b | P | Harrell's C statistic |
| Previous ischemic heart disease | 1.461 (1.281-1.666) | 1.148 (0.985-1.342) | .075 | 0.802 |
| Peripheral arterial disease | 2.498 (2.047-3.049) | 1.570 (1.237-1.978) | <.001 | |
| Cerebrovascular disease (stroke) | 2.006 (1.662-2.422) | 1.342 (1.122-1.699) | .001 | |
| No diseased vascular territories | ||||
| 1 territory | 1.575 (1.379-1.800) | 1.152 (0.897-1.523) | .282 | |
| ≥ 2 territories | 3.819 (3.083-4.749) | 2.349 (1.605-3.644) | <.001 |
HR, hazard ratio; 95%CI, 95% confidence interval.
Adjusted by age, sex, body mass index, family history of ischemic heart disease, diabetes mellitus, hypertension, hyperlipidemia, active smoking, renal failure, neoformation, chronic obstructive pulmonary disease, baseline New York Heart Association functional class ≥2, previous atrial fibrillation, absence of chest pain at presentation, heart rate and systolic arterial pressure at admission, Killip class at admission > 1, heart failure during the clinical course, complete atrioventricular block, angina after infarction/reinfarction, mechanical complication during hospitalization, treatment at discharge (salicylates, thienopyridines, angiotensin-converting enzyme inhibitors, beta blockers, lipid-lowering agents, acenocoumarol), reperfusion during hospitalization, and left ventricular ejection fraction. Additional adjusting by reperfusion time did not substantially change the results.
Schoenfeld residuals test (proportional hazards assumption), chi-square = 29.08; P = .745.
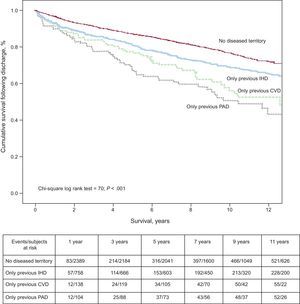
The long-term survival curve calculated separately for each affected territory is shown in Figure 2. In our study, the two vascular territories associated with the greatest post-hospitalization risk of death (Figure 2) were PAD and CVD. Involvement of both territories was documented in 572 patients. The fully adjusted HR for mortality in patients with a history of disease in both territories was 1.518 (95%CI, 1.272-1.812).
DISCUSSIONThis study shows for the first time that in patients admitted to hospital with a diagnosis of STEAMI, a history of IHD, PAD, or CVD is not an independent risk factor for in-hospital death compared to patients with no known vascular disease. At long-term (median, 7 years), a history of previous PAD or CVD was predictive of mortality, and previous IHD was of borderline significance.
It has long been recognized that patients with atherosclerosis have a single disease process, whose extension --rather than “location”-- itself can have an influence on the patient's prognosis.14 Several studies have evaluated the prognostic impact of previous PAD and/or CVD separately4,6,7,10–12 and jointly.3,8,9 Previous myocardial infarction has also shown an important prognostic impact.4,15 A single article9 has reported the prognostic value of previous PAD, CVD, and IHD jointly in relation to therapeutic management and the development of adverse in-hospital events. However, we have not encountered previous studies that evaluate the history of disease at these arterial territories and its implications following discharge at long-term, as is reported here.
In a study performed in Spanish ACS patients from the multicenter MASCARA registry, Ferreira-González et al8 reported that the presence of PAD determined more extensive coronary disease and led to greater mortality during hospitalization and at 6 months. Furthermore, the treatments recommended in related guidelines were less rigorously applied. These authors also reported that a history of CVD was associated with greater mortality at 6 months following discharge, but not with greater in-hospital mortality, in keeping with our results. We highlight several differences between that study and ours which, in our view, may have contributed to certain differences in the findings, particularly related to previous PAD and in-hospital mortality. First, the MASCARA registry included all patients with ACS, with or without ST-segment elevation and of unspecified location. Patients with ACS and ST-elevation, the focus of the study, accounted for about 40% of the total. Second, as is mentioned above, patients in whom stress testing was indicative of myocardial ischemia and those with a known history of coronary disease were considered candidate participants according to the inclusion criteria for the MASCARA study.8 In contrast, all patients without exception in the present study had ST-segment elevation and increased markers of myocardial injury, which makes our population more homogeneous and at substantially higher cardiac risk, as demonstrated by their higher in-hospital and one-year mortality compared to the patients included in the MASCARA registry.
The increased risk of adverse events in patients with a history of CVD has been described in previous studies.7,10,11 A background of CVD has been associated with a higher risk of death at 6 months10 (adjusted OR = 1.41; 95%CI 1.17-1.70), a finding that is consistent with our results and those of the MASCARA study.8 Our study additionally brings to light the adverse impact of previous CVD at long-term.
The importance of previous IHD has also been evaluated in the literature.4,15 In a classic study15 in STEAMI patients, previous myocardial infarction was an adverse prognostic factor at 30 days. Another study4 reported that in-hospital mortality did not differ, but 6-month mortality was higher in patients with previous myocardial infarction than in those without this background. In our study, a history of IHD was associated with higher crude mortality during hospitalization and at long-term follow-up, although it was not an independent predictive factor in the adjusted analysis. The results did not differ when previous myocardial infarction was considered instead of previous IHD.
In our study, vascular burden, defined as the number of affected vascular territories, was predictive of post-hospitalization mortality at long term, but not of in-hospital death. These findings contrast with those of another study,3 in which an association was found between the number of diseased territories and death during hospitalization. However, the patients included differed from those in our study: the population was at lower risk than ours, as indicated by a much lower in-hospital mortality rate (5.3% vs 12.3%), there were fewer patients with diabetes than in our series, and those with STEAMI accounted for < 11%. In another previous study,9 involvement of ≥2 vascular territories was an independent predictor of in-hospital mortality, but the population was comprised of patients with non—ST-segment elevation myocardial infarction. In a sub-study of this last work,16 performed in patients > 65 years, involvement of all 3 territories was associated with elevated mortality at 3 years’ follow-up.
Few studies have evaluated the long-term impact of polyvascular disease. In the important work by Bhatt et al,17 with data from the REACH registry, previous vascular burden had a negative impact on the development of adverse events at long-term (4 years), including all-cause death. Our present findings complement and expand the results of that study, which was performed in stable patients with cardiovascular risk factors or previous vascular disease. Our findings uphold the importance of vascular burden as a predictor of mortality at long-term (> 7 years) in a population with STEAMI.
In line with the results of previous publications,3–8 we also found that patients with a larger number of affected vascular territories receive treatment with salicylates and beta blockers less often, although they are prescribed angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and acenocoumarol more frequently. Thus, paradoxically, these patients have higher mortality, but are prescribed fewer drugs associated with a better prognosis. Based on this fact, we suggest that actions should be taken to optimize their treatment. These observations concur with the results of the notable Alliance project18 in the French population, which concluded that specific studies should be designed to improve the prognosis of this patient subgroup.
LimitationsWe should point out the following limitations of our study: a), there are the drawbacks inherent to observational studies, such as unadjusted bias. Our study is prospective, however, and it includes all consecutive patients from 2 centers; b), the diagnosis of previous IHD, PAD, and CVD is based on clinical criteria and it cannot be ruled out that some asymptomatic or very mild cases might have been wrongly classified. In this regard, Morillas et al19 have indicated that the prevalence of PAD is very high when the ankle-brachial index is used to diagnose this condition in ACS patients; hence the diagnosis of PAD may have been underestimated in our study; c), with respect to some studies that have previously reported an association between vascular burden and in-hospital mortality,3,9 the relatively low number of events during hospitalization may have been the reason why previous vascular burden did not show an association in the adjusted analysis and d) lastly, a history of CVD only included stroke, but not transient ischemic attack or mild CVD.
CONCLUSIONSWe conclude that in patients with a diagnosis of STEAMI, the previous vascular burden is associated with greater mortality at long-term following discharge. A history of PAD and CVD are independent markers of elevated risk at long-term.
CONFLICTS OF INTERESTNone declared.