Recent years have witnessed an explosion in the publication of cardiology clinical trials, to the extent that it is now almost impossible for physicians in our field to keep abreast of even the most important studies.
In interventional cardiology in particular, a constant stream of breakthrough studies has reported major advances in ischemic heart disease, antithrombotic drug therapy, stents, intracoronary diagnosis, high-risk population groups, specific lesions, and—of course—the innovative field of structural therapy, resulting in changes to clinical practice and ushering in modifications to treatment recommendations.
Nonetheless, some studies stand out due to their clinical relevance and their impeccable methodology and above all because they change established working practice. Studies in this category become familiar to everyone working in the field, and their results are applied universally and systematically in routine clinical practice. Without question, the LEADERS FREE study1 belongs to this select group of Olympian studies. This study brought an end, after more than 25 years, to the indication for placement of conventional bare-metal stents. Published at the end of 2015, the LEADERS FREE study randomized 2466 high bleeding risk patients to revascularization with a bare-metal stent or with the BioFreedom drug-coated stent, a polymer-free stent that releases biolimus A9 (Biosensors Europe, Switzerland). The patients received just 1 month of dual antiplatelet therapy. As was to be expected, 1-year follow-up showed the drug-eluting stent to be more effective, but the breakthrough finding was that it was also safer than the bare-metal stent. The BioFreedom drug-eluting stent significantly reduced the main outcome measure (a composite of cardiac death, myocardial infarction, or stent thrombosis) from 12.9% with the bare-metal stent to 9.4% (hazard ratio [HR]=0.71; 95% confidence interval [95%CI], 0.56–0.91: P=.005 for superiority). Bare-metal stents previously held on to a niche role as the treatment for high bleeding risk patients requiring time-limited dual antiplatelet therapy. But with the LEADERS FREE study, even this role was taken over by drug-eluting stents, and the new use of these devices rapidly expanded into routine clinical practice. For example, in Spain the use of drug-eluting stents increased from 79% in 2015 to 94% in 2018.2 Since publication of the study, several Spanish autonomous communities began to use drug-eluting stents in 100% of revascularization procedures, and this pattern is certain soon to spread to all other regions.
A High Bleeding Risk PopulationThe LEADERS FREE study stands out from the pack not only for its breakthrough finding, but also because of its bold study design. The population examined in the study is one of the most challenging for percutaneous coronary intervention (PCI); indeed, high bleeding risk patients are systematically excluded from most studies. Patients in this population tend to be elderly and have multiple comorbidities and more coronary disease, all of which combine to make PCI more complex. Added to this is the need to reduce iatrogenic risk by using less effective antiplatelet regimens, a severe limitation that affects both the intensity and duration of drug therapy.
The prognostic impact of bleeding complications is well known. The mid-term mortality of patients who have major bleeding episodes after PCI can be similar to that of patients with reinfarction. A more detailed picture is provided by the TRACER study of patients with non–ST-segment elevation acute coronary syndrome; this study showed that the mortality risk of bleeding graded >3b according to Bleeding Academic Research Consortium (BARC) criteria is higher than that associated with myocardial infarction.3 This finding was confirmed in a large Spanish cohort of 4299 patients with any type of acute coronary syndrome.4 The authors of the Spanish study also found that mortality risk after a bleeding event was lower in patients on dual antiplatelet therapy than in patients not receiving this treatment.
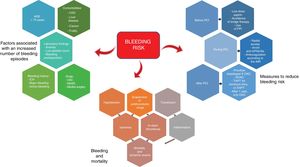
The negative impacts of bleeding are manifold, and prominent among the factors associated with bleeding risk are hypovolemia, the withdrawal of antithrombotic therapy, and the need for blood transfusions (figure 1).
mportant variables in high bleeding risk patients treated by percutaneous coronary intervention. Brown shading, consequences of bleeding. Green shading, factors associated with increased bleeding. Blue shading, measures for reducing bleeding risk. anti-GPIIb/IIIa, glycoprotein IIb/IIIa inhibitor; CKD, chronic kidney disease; DAPT, dual antiplatelet therapy (OAC plus an antiplatelet drug); DOAC, direct oral anticoagulant; ICH, intracranial hemorrhage; INR, international normalized ratio; NSAID, nonsteroidal anti-inflammatory drug; OAC, oral anticoagulant; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor; TAPT, triple antiplatelet therapy (OAC plus 2 antiplatelet drugs).
There is thus a growing and fully justified interest in high bleeding risk patients, reflected in a specific section dedicated to this patient subgroup in the latest European guidelines on dual antiplatelet therapy. The guidelines recommend shorter treatment regimens for the different categories of acute coronary syndrome, albeit at evidence levels B or C.5 The definition of high bleeding risk in patients undergoing PCI has also been the subject of a recent consensus document.6 The definition proposed by the expert panel is a BARC 3 or 5 bleeding risk of ≥ 4% at 1 year or a risk of intracranial hemorrhage ≥ 1% at 1 year. But what is especially interesting is the authors’ consensus definition of 20 major or minor criteria for diagnosing high bleeding risk at the time of PCI; patients are considered to be at high bleeding risk if they meet at least 1 major or 2 minor criteria. The most prevalent of the major criteria are the need for oral anticoagulation, the presence of anemia, chronic kidney disease, and a personal history of severe bleeding. Sadly, high bleeding risk patients often meet several of these criteria, ratcheting up their risk and underlining the urgent need to maximize all measures to improve prognosis.
Measures To Reduce Bleeding RiskThe first step in the treatment of high bleeding risk patients is to determine which risk factors are present. Some bleeding risk factors are intrinsic to the patient, and are thus unmodifiable (eg, age, female sex, low body weight). However, other risk factors are modifiable (eg, antithrombotic therapy and vascular access route) or potentially modifiable (eg, kidney function and baseline hemoglobin).7
The reduction of bleeding risk is one of the most important considerations during cardiac catheterization or PCI (figure 1). Recommendations to reduce this risk should be followed before, during, and after the procedure,8 which requires fluid communication between the clinical cardiologists responsible for patient management and the catheterization specialists carrying out the procedure. Many of these measures are initiated before catheterization and are enormously important for the reduction of bleeding risk. Some measures have historically provoked disagreement between clinicians and interventional cardiologists, such as not suspending oral antiplatelet therapy and, above all, avoiding bridge therapies with low molecular weight heparin. It is therefore important for all practitioners to reach a consensus on how to improve the prognosis of this ever-complex patient subgroup.
The recommended measures to reduce bleeding risk address fundamental questions. a) Key measures preceding catheterization include risk assessment, treatment of modifiable factors such as high blood pressure, the avoidance of bridge therapies in patients on long-term oral anticoagulant therapy, and treatment with proton pump inhibitors. b) During catheterization or PCI, important measures are radial artery access, avoiding the use of glycoprotein IIb/IIIa inhibitors, and selecting anticoagulant therapy during PCI according to anticoagulant type and activity.9c) Key post-PCI measures include limiting the duration of triple therapy as much as possible (or opting for dual therapy if bleeding risk exceeds ischemic risk), using clopidogrel as the antiplatelet drug of choice, and prioritizing the use of direct acting anticoagulant drugs.
Communication between interventional and clinical cardiologists remains critical after PCI. The choice of the post-PCI antithrombotic regimen must be determined by careful assessment not only of the bleeding risk, but also of the ischemic risk that provoked the revascularization in the first place. This assessment requires knowledge of the coronary anatomy, the number of vessels treated, the number of stents implanted, their length, and the technical complexity of the revascularization procedure (eg, bifurcations, chronic occlusions, calcified lesions). It is of course also essential to detect and characterize suboptimal outcomes after PCI. The written transmission of all this information is often complicated and is always incomplete. Clinical and interventional cardiologists should therefore select the post-PCI antithrombotic regimen for a high bleeding risk patient through joint review of the patient's coronary anatomy, the revascularization procedure complexity and outcome, and all previously recorded clinical characteristics.
The Importance Of Radial AccessAnother key consideration with high bleeding risk patients is the importance of vascular access via the radial artery. In most populations and clinical contexts, this access route is associated with a lower rate of bleeding complications, resulting in it becoming the preferred PCI route in most patients. This tendency is affirmed by European coronary revascularization guidelines, which recommend radial artery access as the standard procedure (class I, evidence level A) except in specific circumstances indicating an alternative approach.10 Spain was a committed pioneer in promoting generalized use of radial access. A review of 2018 registry data by the Spanish Society of Cardiology Hemodynamic Section shows that radial access was used in 87.4% of diagnostic procedures and, more importantly, in 89.4% of coronary interventions.2
Against this background, it is pleasing to read the important update provided in a recent study published in the Revista Española de Cardiología,11 in which Jiménez Díaz et al. underline the importance of prioritizing radial artery access for PCI in high bleeding risk patients. In a predefined subanalysis of high bleeding risk patients in the LEADERS FREE clinical trial,1 the authors examine the association between the PCI access route and subsequent major bleeding episodes after 30 days and over a long-term follow-up of 2 years. The information provided in the study is important because until now the impact of the vascular access route has not been reported in this population. The analysis shows that radial access reduces the number of major bleeding episodes after PCI at 30 days (HR=1.98; 95%CI, 1.25-3.11; P=.003) and at 2 years (HR=1.51; 95%CI, 1.14-2.01; P=.003). This beneficial effect is due to reductions in bleeding episodes both related and unrelated to the vascular access route. Moreover, the study by Jiménez Díaz et al. shows that the difference in efficacy and safety between the drug-eluting and bare-metal stents is independent of the vascular access route and that its magnitude is maintained.
The association of radial access with a lower incidence of bleeding episodes unrelated to the access route likely reflects differences in baseline characteristics between patients in the radial and femoral access groups (the LEADERS FREE study population was randomized not for vascular access, but for placement of the drug-coated BioFreedom stent or a bare-metal stent). It is also important to note that the LEADERS FREE population had a much higher incidence of bleeding episodes unrelated to the vascular access route than reported in previous studies. The authors rightly suggest that this reflects the high prevalence of comorbidities and frailty in this elderly population and the need for a high proportion of these patients to receive chronic oral anticoagulation.
The main cause of major bleeding is gastrointestinal hemorrhage, and gastrointestinal bleeding accounted for > 50% of major bleeding events unrelated to the access route in the study population. This is especially interesting given that gastrointestinal bleeding is a frequent consequence of antithrombotic therapy, a key variable in high bleeding risk patients that can be modified to improve prognosis.
The key factor in the choice of antithrombotic therapy for this patient subgroup is the safety of current drug-eluting stents, since this makes it safe to reduce dual antiplatelet therapy. Nevertheless, physicians still face an unresolvable dilemma. Should we curtail antithrombotic therapy to reduce bleeding risk, thus increasing the risk of ischemic events, or should we prioritize ischemic protection with prolonged antithrombotic regimens, knowing that this will increase the number of iatrogenic bleeding episodes?
This is the situation we find ourselves in today, guided by expert recommendations and clinical and interventional cardiologists’ meticulous assessments of all major variables, and above all relying on our own judgement. More solid answers will come in the near future with the publication of results from clinical trials in high bleeding risk patients. The MASTER DAPT12 study in particular will provide crucial information for defining the best antithrombotic regimen in this population. MASTER DAPT randomly assigned short or prolonged antithrombotic therapy to 4300 high bleeding risk patients free of events in the first 30 days after PCI. The primary endpoint is the net benefit in a composite of all-cause mortality, infarction, stroke, and BARC 3 or 5 bleeding. The results are eagerly awaited.
As Cicero said, “The greater the difficulty, the greater the glory.” And it is the high bleeding risk population—difficult, complex, and with poor prospects—that without doubt stands to benefit most from changes to improve prognosis.
We must take all necessary measures to reduce bleeding complications in this highly vulnerable population, and this can only be achieved through partnership between clinical and interventional cardiologists. High bleeding risk patients treated by PCI constitute one of the most afflicted patient populations, and we must improve their prognosis. This is an achievable goal.
Conflicts Of InterestJ.M. Ruiz-Nodar has received payments for speaking engagements from AstraZeneca, Biosensor, Boston Scientific, Medtronic, and Terumo.