Chagas disease is a prevalent cause of heart failure in Latin America, and its prognosis is worse than other etiologies. The Heart Failure Survival Score has been used to assess prognosis in patients with heart failure; however, this score has not yet been studied in patients with Chagas cardiopathy.
MethodsThe Heart Failure Survival Score was calculated in 55 patients with severe left ventricular systolic dysfunction due to Chagas disease. Correlations were assessed between the Heart Failure Survival Score and variables obtained from echocardiograms, cardiopulmonary exercise tests, quality-of-life measures, and 6-minute walking tests.
ResultsPatients were distributed among New York Heart Association classes II–IV; 89% were taking angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, 62% were taking beta-blockers, 86% were taking diuretics, and 74% were taking aldosterone receptor blockers. The mean Heart Failure Survival Score was 8.75 (0.80). The score correlated well with cardiopulmonary test variables such as peak oxygen uptake (0.662; P<.01), oxygen uptake at the anaerobic threshold (0.644; P<.01), ventilation carbon dioxide efficiency slope (−0.417; P<.01), oxygen pulse (0.375; P<.01), oxygen uptake efficiency slope (0.626; P<.01), 6-minute walking test (0.370; P<.01), left ventricle ejection fraction (0.650; P=.01), and left atrium diameter (−0.377; P<.01). There was also a borderline significant correlation between the Heart Failure Survival Score and quality of life (−0.283; P<.05).
ConclusionsIn heart failure patients with Chagas disease, the Heart Failure Survival Score correlated well with the main prognostic functional test variables.
Keywords
.
IntroductionHeart failure (HF) is the end stage for almost all diseases that affect the heart. Despite progress in primary and secondary prevention of heart disease, HF is still a prevalent cause of mortality and hospitalization.1
Several tools have been studied to assess HF prognosis. Scores and the cardiopulmonary exercise test (CPX) are the most established. The Heart Failure Survival Score (HFSS) is one of the most-studied and most-utilized scores in clinical practice.1, 2, 3 CPX is the best and most reproducible way to measure functional capacity and, by interpreting other variables obtained in the same test, the pathophysiology of patient symptoms can also be assessed. Based on the knowledge that HF is a multifactorial syndrome, the HFSS combines data from different prognostic markers in HF to better assess risk in this specific population.
Chagas disease is a prevalent cause of HF in Brazil and other Latin American countries.4 Imported cases are increasing in North America and Europe. The prognosis for HF that is due to Chagas disease seems to be worse than other etiologies.5, 6 A specific score for prognostic assessment in Chagas disease has recently been published.7
Although CPX and its variables (peak oxygen uptake [VO2], ventilatory carbon dioxide efficiency [VE/VCO2 slope], heart rate recovery at the first minute, oxygen uptake efficiency slope [OUES], and oxygen pulse) and the 6-minute walking test (6MWT) have all been vastly studied in HF,8, 9, 10, 11, 12, 13 HFSS performance in HF due to Chagas disease has not been evaluated. Thus, the aim of this study was to assess the correlation between the HFSS, a more generic HF score, and classic prognostic tests for HF in a population with symptomatic and advanced HF due to Chagas disease.
Methods Study PopulationWe studied 55 patients with HF due to Chagas disease. Patients had to be aged 18-75 years and have a Chagas serology confirmation by two distinct methods, as well as severe left ventricular dysfunction (ejection fraction <35%) and symptoms (New York Heart Association [NYHA] classes II–IV) after a period of stable drug therapy. The population comprised consecutive triage patients who fulfilled the above criteria and made all the specific exams, as listed below, before their randomization into one of two distinct trial protocols that were being conducted on Chagas HF patients in our institution.14 From the total, 21 patients were selected for a trial that randomized patients to a treatment with autologous intracoronary stem cells or placebo; the remaining 34 patients were selected for a protocol that randomized patients to the use of pentoxifylline or granulocyte colony-stimulating factor or placebo. Both protocols were looking for improvements in left ventricular function. Patients with pulmonary diseases, primary heart valve disease, coronary obstruction lesions >50%, or those noncompliant with the drug treatment were not selected. Patients completed, as part of the entry exams for both protocols, the following exams: CPX, laboratory tests, electrocardiograms, 24-h Holter monitoring, 6MWT, clinical evaluation, and quality-of-life assessed by the Minnesota Living with Heart Failure Questionnaire (MLHFQ). The study protocol was approved by an institutional review board, and all patients gave informed consent before entry.
Initial EvaluationThe initial evaluation of these patients included a clinical exam performed in all patients by the same physician. At this point, clinical and physical data were collected and NYHA class was determined using the HF-specific Goldman's specific activity scale. Blood samples for laboratory tests were collected after 12h fasting. Electrocardiograms were performed with the patients in supine position after 5min of rest. Two-dimensional color Doppler echocardiograms were performed on all patients by the same operator, ejection fractions were assessed using the Simpson method, and classic M-mode measures were determined. Then the Holter electrocardiogram monitoring was scheduled. The monitors were placed in the morning and removed the following morning. Patients were instructed to maintain their daily activities and medications during the recording period. The 6MWT was performed, by the same physician, using a finger pulse oximeter in a 30-meter-long hallway; the patient was instructed to walk in his or her most intense rhythm for 6min, and the distance walked was determined for each patient. Also, the MLHFQ was completed by each patient.15
Cardiopulmonary Exercise Test ProceduresSymptom-limited CPX were performed on a treadmill with a breath-by-breath gas analyzer (Cortex MetaLyzer 3B; Cortex Biophysik, Leipzig, Germany) that was calibrated with gas before each test. An individualized ramp protocol was used to match a duration of 8 to 12min for the exercise phase. Electrocardiograms were recorded at rest and in at least 2-min intervals during the exercise. Electrocardiogram monitoring was continued for at least 4min of the recovery phase. Arterial pressure was verified at rest and at each minute throughout the entire exam. Ventilatory data collected breath-by-breath were tabulated in 10-s intervals. Peak VO2 and exchange ratio were determined as the peak values achieved during the exercise phase. Anaerobic threshold was determined by the V-slope method.16 The VE/VCO2 slope was calculated based on the ventilation (VE) and CO2 production (VCO2) from the beginning of the exercise through the peak exercise, as in the linear regression model (y=mχ+b, where m=slope). Based also upon previous publications, the cut point of >34 was used as a determinant of worse outcome for that variable.9 The same data from VE and VO2 in that same interval were used to determine the OUES using previously published logarithmic equations.17 Heart rate recovery at the first minute was determined as the difference between: a) heart rate at peak exercise, and b) heart rate at the first minute of passive recovery. Based upon previous publications in HF populations, a cut point of <16 bpm was used as a risk marker.11 Oxygen pulse was calculated as the ratio between peak VO2 and peak heart rate and expressed as mL/beat.
Heart Failure Survival ScoreThe HFSS was calculated in accordance to its original publication using the variables inherent for each patient collected at the initial visit and basal exams.3, 7
HFSS=[(0.0216×rest heart rate)+(–0.0255×mean arterial pressure)+(–0.0464×ejection fraction)+(–0.047×serum sodium)+(–0.0546×peak VO2)+(0.608×presence of interventricular conduction defect £)+(0.6931×presence of coronary arterial disease £)].
£ present=1 or absent=0
Statistical AnalysisData were expressed as mean (standard deviation). Pearson correlation coefficients were used for association between variables. The patient population was divided into groups based on the peak VO2 (peak VO2 ≤12mL/kg/min and peak VO2 >12mL/kg/min). Specific HF guidelines use this as a worse prognosis cut point for peak VO2 in those treated with beta-blockers.2 The HFSS values, CPX variable results, electrocardiogram variables, MLHFQ scores, and 6MWT results were compared between the 2 groups. The chi-square test was used for categorical variables, and the unpaired t-test was used for continuous variables. A P value <.05 determined significance.
Results Baseline CharacteristicsTable 1 shows the demographic, clinical, and laboratory characteristics of the population. The majority of the patients were male (69%). Also, 89% of patients used angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists and 62% used beta-blockers. The mean value for the HFSS was 8.75 (0.80). Among the variables of this score, the mean values for sodium, mean arterial pressure, ejection fraction, and peak VO2 were as follows: 138 mEq/L, 78mmHg, 27.6%, and 17.3mL/kg/min, respectively. Intraventricular conduction defect or paced rhythm was present in 47 patients (85%). No patients had coronary artery disease. The mean distance in the 6MWT was <400 m. The mean value for the MLHFQ was 38.
Table 1. Baseline Characteristics and Medications.
| Total population, no. | 55 |
| Male sex, no. (%) | 38 (69) |
| Age, years | 52±9 |
| NYHA class, no. (%) | |
| I | 0 (0) |
| II | 41 (74) |
| III | 12 (22) |
| IV | 2 (4) |
| Rhythm, no. (%) | |
| Sinus | 40 (73) |
| Atrial fibrillation | 6 (11) |
| Pacemaker | 9 (16) |
| Clinical data | |
| Systolic pressure, mmHg | 104±16 |
| Mean arterial pressure, mmHg | 78±13 |
| Heart rate, bpm | 68±8 |
| BMI | 24±3 |
| Medication, % in use | |
| ACE inhibitors | 60 |
| ARBs | 29 |
| Beta-blockers | 62 |
| Diuretic | 86 |
| Digital | 69 |
| Aldosterone inhibitor | 74 |
| Laboratory data | |
| Hemoglobin, g/dL | 14±1 |
| Hematocrit, % | 42±4 |
| Sodium, mEq/L | 138±4 |
| Potassium, mEq/L | 4.5±0.4 |
| Urea, mg/dL | 46±20 |
| Creatinine, mg/dL | 1.2±0.3 |
| MLHFQ score | 38±18 |
| 6-min walk distance, m | 399±102 |
| QRS duration, min | 132±21 |
| LBBB or RBBB or paced, % | 85 |
| Ventricular tachycardia detected on Holter, % | 78 |
| Low QRS voltage, % | 11 |
| HFSS, range | 8.75±0.80 (7.05–10.69) |
| Chagas score, range | 13±2 (8–20) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; BMI, body mass index; HFSS, Heart Failure Survival Score; LBBB, left bundle branch block; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; RBBB, right bundle branch block.
Data are expressed as mean±standard deviation or percentages, unless otherwise noted.
Echocardiography data are shown in Table 2. The mean ejection fraction of 27.6% and mean left atrium diameter of 44 mm characterizes a group with severe cardiac dysfunction and volume overload.
Table 2. Echocardiogram Mean Values for the Total Population (n=55).
| Variables | Mean values |
| Ejection fraction, % | 27.6 (6.6) |
| LA diameter, mm | 44 (7) |
| LV final systolic diameter, mm | 60 (9) |
| LV final diastolic diameter, mm | 68 (8.7) |
LA, left atrium; LV, left ventricle.
Data are expressed as mean (standard deviation).
CPX data are shown in Table 3. The mean exchange ratio was 1.08, which was compatible with a maximal exercise for the metabolic point of view (exchange ratio >1.05). The mean peak VO2 was 17.3mL/kg/min, compatible with a mean Weber et al.18 classification of II/IV. The mean VE/VCO2 slope was 36 (>34). The mean percentage of the O2 pulse was 74%. It is important to note that 61% of patients had an attenuated heart rate recovery of <16 bpm.
Table 3. General Cardiopulmonary Exercise Test Variable Characteristics (n=55).
| Variables | Values |
| VO2, mL/kg/min | 17.3 (6.2) |
| Absolute VO2, L/min | 1.09 (0.47) |
| RER | 1.08 (0.17) |
| VE/VCO2 slope | 36 (10.6) |
| OUES, L/min | 0.66 (0.27) |
| Peak O2 pulse, mL/beat | 9.3 (3.5) |
| Peak O2 pulse achieved, % | 74 (20) |
| VO2 at anaerobic threshold, mL/kg/min | 11.6 (3.4) |
| HR recovery <16 bpm, % | 61 |
HR, heart rate; OUES, oxygen uptake efficiency slope; VO2, peak oxygen uptake; RER, exchange ratio; VE/VCO2 slope, ventilatory carbon dioxide efficiency.
Data are expressed as mean (standard deviation) or percentages.
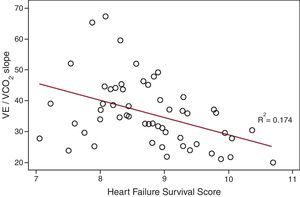
Table 4 shows the correlations between HFSS and HF prognostic variables and the related beta and alpha regression coefficients. The HFSS was associated with the CPX variables (peak VO2, VO2 in the anaerobic threshold, VE/VCO2 slope (Figure), OUES, and O2 pulse, 6MWT, ejection fraction, left atrium diameter, and MLHFQ score). Table 5 shows the comparison within groups for peak VO2 >12mL/kg/min or peak VO2≤ 12mL/kg/min. There was a significant difference in the HFSS, CPX variables, quality-of-life measurement, and 6MWT results between groups.
Table 4. Correlations Between Prognostic Variables and Heart Failure Survival Score.
| Variable | HFSS (Pearson correlation) | Regression coefficients | |
| α | β (95%CI) | ||
| VO2, mL/kg/min | 0.662 a | 7.2 | 0.09 (0.06-0.11) |
| VO2 at anaerobic threshold, mL/kg/min | 0.644 a | 7.0 | 0.15 (0.10-0.20) |
| VE/VCO2 slope | −0.417 a | 9.9 | –0.03 (–0.05 to –0.01) |
| O2 pulse | 0.375 a | 7.9 | 0.08 (0.03–0.14) |
| OUES, L/min | 0.626 a | 7.5 | 1.8 (1.1-2.4) |
| 6MWT, m | 0.370 a | 7.6 | 0.003 (0.001-0.005) |
| Ejection fraction, % | 0.650 a | 6.5 | 0.08 (0.05-0.1) |
| LA diameter, mm | −0.377 a | 10.7 | –0.04 (-0.07 to –0.10) |
| QOL | −0.283 b | 9.2 | –0.01 (–0.02 to –0) |
6MWT, 6-min walking test; 95%CI, 95% confidence interval; HFSS, Heart Failure Survival Score; LA, left atrium; OUES, oxygen uptake efficiency slope; QOL, quality of life; VE/VCO2 slope, ventilatory carbon dioxide efficiency; VO2, peak oxygen uptake.
a P<.01.
b P<.05.
Figure. Scatter plot graphic representation of the correlations between Heart Failure Survival Score and ventilatory carbon dioxide efficiency, VE/VCO2 slope.
Table 5. Comparison Between Groups for Peak Oxygen Uptake.
| Variable | VO2≤12 mL/kg/min (n=9) | VO2>12 mL/kg/min (n=46) | P |
| VE/VCO2 slope | 45.3 (13) | 34.2 (9) | .003 |
| RER | 0.94 (0.16) | 1.10 (0.16) | .011 |
| O2 pulse, mL/beat | 6.7 (2.6) | 9.9 (3.4) | .012 |
| VO2 at anaerobic threshold, mL/kg/min | 7.4 (1.8) | 12.5 (3) | .001 |
| OUES | 0.45 (0.23) | 0.71 (0.26) | .008 |
| Ejection fraction, % | 25 (4.5) | 28 (6.9) | .191 |
| 6MWT, min | 279 (66) | 415 (96) | .002 |
| QOL | 55 (13) | 35 (17) | .002 |
| HFSS | 7.82 (0.45) | 8.93 (0.73) | .001 |
6MWT, 6-minute walking test; HFSS, Heart Failure Survival Score; OUES, oxygen uptake efficiency slope; QOL, quality of life; RER, exchange ratio; VE/VCO2 slope, ventilatory carbon dioxide efficiency; VO2, peak oxygen uptake.
Data, except for P values, are expressed as mean (standard deviation).
Our study focused on the analysis of the HFSS in patients with HF and Chagas disease. Importantly, we described the correlations between the HFSS and other prognostic variables of HF in a specific population of Chagas HF and severe systolic dysfunction. Our cohort reflected a group of severe symptomatic Chagas disease HF patients with a mean ejection fraction of 27.6% and mean peak VO2 of 17.3mL/kg/min. We demonstrated that the HFSS was well associated with the main prognostic variables from the CPX, electrocardiogram, 6MWT, and questionnaire. By stratifying patients using a cut point of ≤12mL/kg/min or >12mL/kg/min for peak VO2, we could demonstrate that those patients with the lower values in the group had a significantly lower HFSS.
The HFSS has been used since its publication to determine HF prognosis and has been validated in cohorts of patients independent of sex, race, and use of beta-blockers.19, 20, 21 It is also an accepted tool for heart transplant decision. Despite its vast use and divulgation, this score had not yet been tested in a Chagas HF population. Although there is already a specific score for HF due to Chagas disease, which includes all forms of Chagas cardiopathy (indeterminate, arrhythmic, and dilated forms), there is no specific score for Chagas patients with HF symptoms. Moreover, as the HFSS is already accepted for other etiologies, if it can also be used in HF due to Chagas it may facilitate the management of these patients in HF clinics, which usually treat patients with HF with different etiologies.
The population of our study represents a group of patients in the HF stages C and D according to American Heart Association guidelines.1 Most patients were in NYHA classes II and III; this is in agreement with the mean peak VO2 of 17.3mL/kg /min, which is compatible with a mean class II/IV of Weber et al. classification.18 Previous studies stratified risk using the HFSS and classified groups as high, medium, and low risk using the respective cut points: <7.20, 7.20–8.09, and >8.09.3 The mean HFSS of our population was 8.75, which is compatible with a low-risk group. Using cut points for peak VO2 (<10, 10–14, and >14mL/kg/min) as determined by Mancini et al.,8 the group would also be set as a low-risk group, showing a similar risk stratification by VO2 and HFSS analysis in this group of Chagas disease patients. On the other hand, Mady et al.22 found a survival rate of only 66% at 1 year in a Chagas HF population with a mean peak VO2 of 18mL/kg/min. This contrasts with the 94% survival rate found by Mancini et al.8 in general HF patients with a peak VO2 >14mL/kg/min.
Koelling et al.,21 who analyzed a group of 500 ambulatory HF patients in the current era of HF treatment with a mean peak VO2 of 15.7mL/kg/min and mean ejection fraction of 21%, found a mean HFSS of 8.01. Compared with patients in Koelling et al. study, our patients had a higher prevalence of intraventricular conduction defect (85% vs 25%). Of note, 56% of patients had ischemic cardiopathy in Koelling et al. study, which may be associated with a worse HFSS. Previous studies have shown that Chagas cardiopathy prognosis is worse than other etiologies, including ischemic.6, 23 The mean heart rate in our patients was 68 bpm, which was lower than the mean of 81 bpm in Koelling et al. study, even though the percentage of beta-blocker use was similar (62% in our population vs 59% in Koelling et al. population). Because of specific characteristics inherent to the Chagas etiology that are related to prognosis and basal heart rate, for example, the cut points for the HFSS in this condition may differ from the general HF population.
We demonstrated that HFSS in Chagas HF patients is associated with the main functional capacity and ventilatory variables of the CPX, the 6MWT, ejection fraction, and quality of life. As the peak VO2 is already part of the HFSS calculation, the association between these variables and with those dependent on the peak VO2 was expected. The same is true for left ventricular ejection fraction. In recent years, many studies have shown the prognostic importance of the VE/VCO2 slope in HF.9, 11 This variable has an independent and even greater prognostic significance than peak VO2. There is a linear correlation between VE and VCO2 during exercise because the increment in dead space related to pulmonary congestion results in an elevation in VE, which in turn leads to a higher value for that ratio. The prognostic significance of this variable is maintained even in submaximal effort. We were able to show an association between the HFSS and the VE/VCO2 slope (Figure). It is important to note that, for the HFSS calculation, we need data from variables acquired from different exams; for the VE/VCO2 slope calculation, all data are derived from a unique CPX. For the development of any score in a certain disease, it is necessary to have a good understanding of the variables and their association with outcomes. All variables are not always readily available in a first appointment to calculate a given score. Therefore, understanding individual variables as well as their correlations with well-validated scores is important and might help decision making, particularly when variables used to calculate the scores are still unavailable to be used in the initial visits.
Mortality is not the only important outcome in HF. Quality of life also assumes an important and relevant role. Some therapies extend life without increasing quality of life. Researchers have studied how quality of life relates to functional variables24, 25, 26 and have come to different conclusions. How HFSS relates to quality of life had not yet been studied. In our population, we were able to show a weak but statistically significant correlation between quality of life (as assessed by the MLHFQ) and the HFSS.
Guidelines accept a peak VO2 of ≤12mL/kg/min as a criterion for heart transplant selection in symptomatic patients with HF using optimized evidence-based medication, including beta-blockers.2 In our study, we split the population into 2 groups of better and worse prognosis based on the peak VO2, and it showed that functional variables and quality of life differed within groups in the same way that HFSS differed in statistical significance between groups. The mean HFSS in the group with peak VO2≤12mL/kg/min was 7.82, compatible with a medium-risk HFSS. The mean 6MWT distance and the VE/VCO2 slope value in the ≤12mL/kg/min group (279 m and 45.3, respectively) were compatible with high risk (<300 m)13 and with a respiratory class of IV as assessed by the VE/VCO2 slope (VE/VCO2 slope>44).11
LimitationsOur study has some limitations. First, the sample size is small. Second, this is a single-center study. Third, we did not collect data on right ventricular function, which has been demonstrated to be of value for predicting functional capacity in Chagas patients;27 thus, we could not test the correlations between HFSS and right ventricular function in this population. Finally, we do not have clinical outcomes that test the correlation between the cardiopulmonary and echocardiographic variables with clinical outcomes.
ConclusionsIn HF patients with Chagas disease, the HFSS correlated well with the main prognostic functional test variables but had a weak correlation with quality of life. As expected, patients with lower functional classes had a lower HFSS. The HFSS might be a useful tool for prognostic assessment in HF due to Chagas disease. Studies with longitudinal and clinical follow-up are required to better determine the optimal thresholds of the HFSS for decision making in this specific population.
FundingThis study was fully funded by the Brazilian Ministry of Health, Foundation for Research Support of the State of Bahia, and the Brazilian Clinical Research Institute in São Paulo, Brazil.
Conflicts of interestNone declared.
Received 20 October 2011
Accepted 22 December 2011
Corresponding author: Duke Clinical Research Institute, Box 3850, 2400 Pratt Street, Room 0311, Terrace Level, Durham, NC 27705, United States. renato.lopes@duke.edu