To the Editor:
Right ventricular arrhythmogenic dysplasia (RVAD) is an uncommon entity characterized by ventricular arrhythmia, heart failure, and sudden death in young individuals.1 Hereditary variants with an autosomal dominant pattern and other mechanisms involved in its etiopathogenesis such as apoptosis and inflammatory processes have been identified, and abnormalities in various genetic loci and intercellular junctional proteins such as plakoglobin and desmoplakin have been described.2 The presence of focal myocarditis is common in the histopathological study of this cardiomyopathy, but the interpretation of this finding is controversial.3 This finding has been explained as myocarditis in which fibroadipose repair is based on structural alteration of the right ventricle (RV), or as a sporadic process that develops because of the greater susceptibility of the dysplastic myocardium in these patients.4
Giant cell myocarditis is a specific autoimmune disease characterized by acute, refractory, often fulminant heart failure, although chronic variants such as idiopathic dilated cardiomyopathy have been described in retrospective studies.5 Although it may respond to combined immunosuppressive therapy, heart transplantation (HT) is often necessary.6 The possibility of recurrence of the disease in a high percentage of patients after HT has been described as characteristic.6
We describe a 38-year-old woman with no family history of heart disease or sudden death, diagnosed 5 years earlier with a cardiomyopathy involving only the RV, with no ventricular arrhythmia observed during the course of the disease. A year earlier, she had been admitted for acute pulmonary edema, at which time she was diagnosed with precirrhosis of the liver due to stasis, with the development of incipient esophageal varices.
The patient was referred to the HT unit and found to be New York Heart Association (NYHA) Class III-IV. The electrocardiogram showed atrial flutter and right bundle-branch block with no epsilon wave. Cardiac magnetic resonance revealed cardiomyopathy with a dilated RV with areas of aneurysms and dyskinesia, particularly in the apex, and moderate-to-severe systolic dysfunction of the left ventricle. Based on the patient's advanced clinical condition, HT was performed.
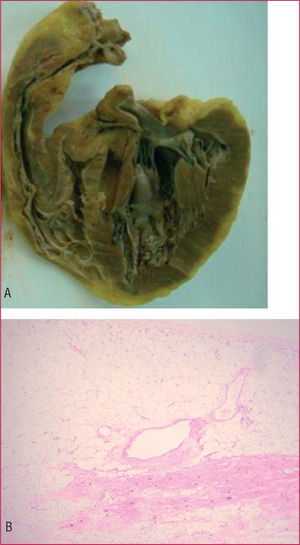
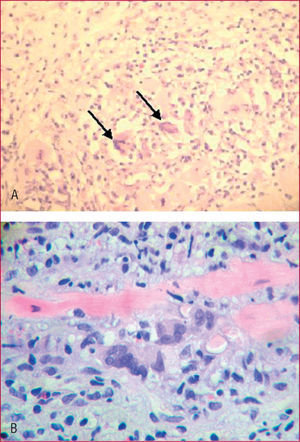
On pathological study of the explanted heart, the RV showed a thinned wall with aneurysmal dilatations and adipose infiltration over 70% of its thickness (Figure 1, A and B). Microscopy disclosed foci of interstitial lymphocytic myocarditis with giant cells in the interventricular septum (Figure 2A).
Figure 1. A: macroscopic image of the right ventricle of the explanted heart showing characteristic thinning and aneurysmal formations of right ventricular arrhythmogenic dysplasia. B: right ventricular wall with predominance of fibroadipose tissue over the muscle fibers.
Figure 2. A: lymphocytic infiltrates and giant cells (arrows) in the septal myocardium of the explanted heart (HE, x200). B: detailed view of inflammatory infiltrate in a post-transplant biopsy. In the center, 3 multinucleated giant cells and a myocyte are evident (HE, x400).
Endomyocardial biopsy 2 months after transplantation showed asymptomatic recurrence of the lymphocytic infiltrate and the presence of giant cells (Figure 2B), which improved following adjustment in the immunosuppressive therapy.
A high percentage of myocarditis with RV involvement alone has been observed in biopsies of patients with no family history and a diagnosis of RVAD based on clinical and imaging evidence; these patients have a more favorable prognosis.3 Post-transplantation recurrence of myocarditis characterized by giant cells and initial improvement with increased immunosuppression is characteristic of this condition; the medium-term to long-term prognosis is inconclusive.5,6
We believe that this case highlights the usefulness of endomyocardial biopsy in cardiomyopathies where RVAD is suspected in the magnetic resonance imaging study, both to identify the characteristic findings and to rule out other entities such as myocarditis with isolated RV involvement. Patchy myocardial involvement is the main limitation of this technique. If a prior histological diagnosis by endomyocardial biopsy had been made in the patient described, empirical immunosuppressive therapy could have been attempted. Because the condition was found after HT, closer follow-up is necessary, given the high probability of recurrence.