Keywords
INTRODUCTION
Adipocytokines are bioactive proteins that are synthesized and secreted by adipose tissue (AT). They play an important role in the regulation of energy metabolism, immune function, vascular remodeling, angiogenesis, and neuroendocrine function.1 These proteins can act locally, through autocrine/paracrine mechanisms, or systemically, through endocrine effects.2
Adiponectin is a 28-kDa protein3 that is synthesized mainly by adipocytes. Its production by other cell types, such as cardiomyocytes, has also been reported.4 This hormone, which circulates in abundance in human plasma, constitutes 0.01% of total plasma proteins.5 One of the major roles of this hormone is to improve insulin resistance by increasing AT fatty acid oxidation and decreasing plasma fatty acid concentrations and intracellular triglyceride accumulation in the liver and muscle. It also plays a protective role in atherosclerotic processes,6 and its concentrations are reduced in states of insulin resistance, such as obesity and type 2 diabetes,7,8 as well as in patients with coronary artery disease.9
Leptin is a 16-kDa cytokine that acts as a saciety signal in the central nervous system and is related to glucose and insulin metabolism. It has been proposed to be the link between obesity, diabetes and cardiovascular risk.10
Serum leptin concentrations increase exponentially with the increase in body fat mass,11 and hyperleptinemia is a reflection of the leptin resistance associated with obesity.12 Patients with heart failure present elevated serum leptin concentrations,13 which have also been associated with a decrease in arterial distensibility14 and with increased inflammatory marker levels in obese individuals.15
Both adipocytokines are secreted in greater amounts by subcutaneous AT than by visceral AT.16,17 It has recently been demonstrated that epicardial AT releases higher proportions of different inflammatory mediators than subcutaneous AT in patients at high cardiac risk.18 Likewise, Iacobellis et al19 detected adiponectin protein expression in epicardial AT, observing lower levels in patients with severe coronary artery disease.19 This finding attributes new functions to epicardial AT, although the significance of the results has yet to be elucidated.
In view of previous studies indicating the antiinflammatory properties of adiponectin20 and the association between leptin and inflammatory markers,15 we proposed to determine whether there was any difference between the epicardial and subcutaneous AT in patients with cardiovascular disease in terms of adiponectin and leptin messenger RNA (mRNA).
METHODS
This study was carried out with the approval of the local ethics committee and the informed consent of all the participating patients. Samples of epicardial and subcutaneous AT were obtained from 46 cardiac surgery patients, 15 women and 31 men, who had undergone coronary artery bypass surgery or aortic, or mitral valve replacement. The tissue was collected prior to cardiopulmonary bypass and was immediately frozen in liquid nitrogen and stored at 80 ºC until processing. As each patient served as his or her own control, only those with some type of viral infection were excluded.
The mean age of the women included was 71.1 (1.3) years, whereas that of the men was 67.4 (1.7) years, and the mean body mass index was 29.5 (1.3) and 28.48 (0.5), respectively. The clinical parameters and features assessed, as well as the medications received by the patients, are shown in Table.
Immunohistochemistry
The qualitative analysis of adiponectin and leptin protein expression was performed in a small portion of the epicardial and subcutaneous AT of a randomly chosen patient.
The tissue samples were fixed in 4% formaldehyde, dehydrated and cut into 5-µm sections.
Antigen detection consisted of incubation with the primary antibody. In the case of adiponectin, a 1/200 dilution of goat anti-ACRP30 N-20 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was employed overnight at 4ºC. For the detection of leptin, the samples were incubated for one hour at room temperature with rabbit anti-Ob A20 antibody (Santa Cruz Biotechnology); the samples were subsequently incubated with the streptavidin-peroxidase complex Dako LSAB® System HRP (Dako) for 30 minutes and developed for 10 minutes with 3,3'-diaminobenzidine tetrahydrochloride (DAB), prepared according to the instructions of the manufacturer.
Negative controls were prepared with the primary antibodies, previously blocked overnight at 4ºC with their respective antigens or using TBS without antibody.
RNA Extraction
RNA was extracted by the homogenization of the AT, under cold conditions, in a sodium citrate solution (0.025 mol/L) composed of guanidine thiocyanate (4 mol/L), beta-mercaptoethanol (0.1 mol/L) and sodium lauroyl sarcosinate (0.5%), followed by isolation using the method described by Chomzynski and Sacchi.21 The RNA concentration was analyzed at an absorbance of 260 nm and the purity of the sample was determined on the basis of the ratio of absorbance at 260 nm to absorbance at 280 nm. The sample was then treated with Dnase for two hours at 37ºC, using 10 U/µL of DNase I and 20 U/µL of RNase inhibitor (both from Invitrogen Ltd., Paisley, UK) for every 5 µg of RNA. The proteins and the DNA were eliminated from the samples by extraction with phenol-chloroform-isoamyl alcohol. The RNA was precipitated with 96% ethanol and sodium acetate (0.3 mol/L) and resuspended in water treated with 0.1% diethylpyrocarbonate.
Real-Time Reverse Transcriptase Polymerase Chain Reaction
First, 1.2 µg of purified RNA were utilized for the synthesis of complementary DNA (cDNA). The retrotranscription reaction was performed using 200 U of the enzyme MMLV reverse transcriptase (Invitrogen) in 20 µL of a solution composed of 20 mmol/L Tris-HCL buffer (pH 8.4), 50 mmol/L KCl, 2.5 mmol/L MgCl2, 1 mmol/L each dNTP, 20 U of ribonuclease inhibitor and hexamer fragments as primers. The reaction conditions were: 50 minutes at 37ºC, 10 minutes at 42ºC and 5 minutes at 95ºC.
The comparative analysis of adiponectin and leptin expression was carried out by means of real-time polymerase chain reaction. The technique was performed according to the instructions of the manufacturer, in 20 µL, using 2 µL of the cDNA obtained from retrotranscription, 0.25 µmol/L of the corresponding primers, 3 mmol/L MgCl2, 2 µL SYBR Green (Roche Diagnostics SL, Barcelona, Spain) and 30 nmol/L Rox (Stratagene, La Jolla, CA, USA). Gene expression was quantified by means of the fluorescence emitted by the fluorochrome after the binding of SYBR Green to the double-stranded DNA. The fluorochrome Rox was utilized as a control for the DNA load in the reaction. A single peak per amplification product in the dissociation curve was considered to indicate purity.
The following primers were employed for the amplification of the adiponectin22 and leptin22 genes:
- Adiponectin
Sense: 5'-TGGTGAGAAGGGTGAGAA-3'
Antisense: 5'-AGATCTTGGTAAAGCGAATG-3'
- Leptin
Sense: 5'-TTGGCCCTATCTTTTCTATG-3'
Antisense: 5'-GCATACTGGTGAGGATCTGT-3'
As a control for the load and quality of the cDNA employed in the study, the gene encoding glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was employed23:
- GAPDH
Sense: 5'-TCCATGACAACTTTGGCATCGTGG-3'
Antisense: 5'-GTTGCTGTTGAAGTCACAGGA-GAC-3'
The gene amplification procedure consisted of denaturation at 95ºC for five minutes, followed by 40 cycles involving denaturation (30 seconds at 95ºC), hybridization (45 seconds at 6ºC) and one minute at 72ºC for DNA extension. The amplification products were separated by electrophoresis on a 1.5% agarose gel, stained with ethidium bromide and visualized by means of the Typhoon 9410 image acquisition system (Amersham Pharmacia Biotechnology, Inc., Freiburg, Germany).
The analysis of the results obtained was carried out using the software installed in the real-time polymerase chain reaction system (Mx3000PTM; Stratagene). For each sample, the software package calculates the number of the cycle in which the reader begins to detect a significant increase in fluorescence with respect to the baseline signal. This cycle is referred to as the cut-off point or threshold cycle. In each sample, the levels of adiponectin and leptin expression were normalized to the expression of the internal control, GAPDH.
Statistical Analysis
The results obtained in this study were expressed as mean value plus or minus the standard deviation (SD). The comparisons between epicardial and subcutaneous tissue from each patient were performed using the paired Student t test. For gender comparisons, unpaired Student t test was employed and the 95% confidence interval was calculated. P values less than .05 were considered to indicate statistical significance. For the statistical analysis, the GraphPad Instat software package was used.
RESULTS
The main clinical features of the 46 patients studies are shown in Table There were no significant differences between men and women in terms of the parameters analyzed. This Table also indicates the number of patients (men and women) receiving the different drug therapies employed in the treatment of cardiovascular diseases and diabetes and, again, the absence of statistically significant differences between the two groups. Only one man was being treated with glitazone (rosiglitazone).
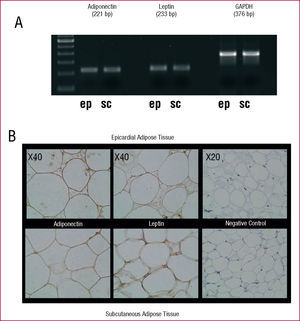
Epicardial and subcutaneous AT express adiponectin and leptin (Figure 1). Amplification products of 221 bp, 223 bp and 376 bp were obtained for adiponectin, leptin, and GAPDH, respectively. The corresponding negative controls showed no evidence of contamination.
Figure 1. A: results of the reverse transcription polymerase chain reaction products in agarose gel for adiponectin, leptin and GAPDH in epicardial (ep) and subcutaneous (sc) adipose tissue. B: immunoreactivity of epicardial and subcutaneous adipose tissue of a patient with antibodies to adiponectin (left) and to leptin (center). The image on the right shows the negative controls.
Immunohistochemical analysis of protein expression revealed that the cytoplasm of the adipocytes in epicardial and subcutaneous AT is immunoreactive for adiponectin, and leptin. There was no staining whatsoever of the corresponding negative controls (Figure 1).
The relative values of the level of expression of adiponectin were higher than those of leptin in both epicardial and subcutaneous AT; however, the levels of adiponectin and leptin mRNA were significantly higher in subcutaneous AT than in epicardial AT. The adiponectin mRNA concentrations were significantly higher in subcutaneous AT (13.54 [0.59] [n=46] versus 12.3 [0.48] [n=46]; P<.05). Likewise, the values obtained for leptin were also significantly higher in subcutaneous AT (9.8 [0.39] [n=46] vs 8.7 [0.26] [n=46]; P<.01) (Figure 2).
Figure 2. Results of quantitative real-time reverse transcription polymerase chain reaction showing the levels of adiponectin and leptin expression in epicardial adipose tissue (EAT) and subcutaneous adipose tissue (SAT) of 46 patients with respect to GAPDH. The superscripts indicate the statistically significant differences (aP<.05; bP<.01).
There was no significant correlation between the clinical features shown in Table (age, body mass index, cholesterol levels, etc.) and the levels of adiponectin or leptin expression. When the levels of adiponectin and leptin expression were analyzed according to sex, we observed statistically significant differences. In the women, the degrees of adiponectin expression in epicardial AT were significantly higher than in the men (14.81 [0.47] [n=15] versus 11.08 [0.55] [n=31]; P<.001) (Figure 3A), whereas there were no differences in subcutaneous AT. The levels of expression of leptin were also higher in epicardial AT of women than in that of men (9.4 [0.4] [n=15] vs 8.3 [0.3] [n=31]; P<.05) (Figure 3B), whereas no differences were observed in subcutaneous AT.
Figure 3. Results of quantitative real-time reverse transcription polymerase chain reaction showing levels of adiponectin (A) and leptin (B) expression in epicardial adipose tissue (EAT) and subcutaneous adipose tissue (SAT) of 31 men and 15 women, who had undergone cardiac surgery, with respect to GAPDH. The superscripts indicate the statistically significant differences (aP<.05; bP<.01; cP<.001).
In men, the differences between epicardial and subcutaneous tissues, which were statistically significant, were maintained both for adiponectin (11.28 [0.59] vs 12.83 [0.61]; P<.01) and for leptin (8.32 [0.33] vs 9.32 [0.36]; P<.01). However, significant differences were not observed between epicardial and subcutaneous AT in women (Figures 3A and 3B).
The ratio of adiponectin mRNA expression to leptin mRNA expression in epicardial AT was significantly higher in women than in men (1.62 [0.1] vs 1.35 [0.04]; P<.01) (Figure 4). As in the results reported above, there were no significant differences in subcutaneous AT (Figure 4).
Figure 4. Ratio of adiponectin mRNA expression to leptin mRNA expression in epicardial adipose tissue (EAT) and subcutaneous adipose tissue (SAT) in men and women. **P<.01.
No statistically significant differences were observed when the adiponectin and leptin mRNA expression in epicardial AT in patients who had undergone coronary bypass (vascular disease) was compared with that of patients who had undergone valve replacement (Figure 5).
Figure 5. Results of quantitative real-time reverse transcription polymerase chain reaction showing adiponectin and leptin expression in epicardial adipose tissue, from 34 patients with ischemic heart disease (IHD) and 17 who underwent valve replacement (VR), with respect to GAPDH.
DISCUSSION
Obesity is a cardiovascular risk factor that not only acts passively by increasing the workload of the heart, but actively as well, by provoking changes in the plasma concentrations of certain molecular factors produced by AT. These factors have cardiovascular effects, as well as a regulatory role in energy metabolism,24-26 circumstances that convert fat into an authentic endocrine organ.
Adiponectin and leptin are among the factors secreted by AT and are included in the group of adipocytokines.1 There is an association between plasma leptin concentrations and the classic cardiovascular risk factors, insulin resistance, the presence of metabolic syndrome and inflammatory markers, even when the proportion of body fat is within normal range.27-29 Plasma leptin concentrations are increased in hypercholesterolemic men, and are associated with higher coronary risk.30
The hormone, adiponectin, is directly related to insulin sensitivity, abdominal obesity and changes in the lipid profile (particularly, low levels of high-density lipoprotein cholesterol [HDL-C]). On the other hand, there is an inverse relationship between vascular inflammation and the plasma levels of this hormone.20 Moreover, a direct relationship between reduced plasma adiponectin concentrations and the extension of atherosclerosis has been observed in a number of studies, and lower levels of this hormone have been documented in men with ischemic heart disease.25
According to a recent report, patients with ischemic heart disease present higher levels of expression of monocyte chemotactic protein (MCP-1) and of certain proinflammatory cytokines (interleukin 1) in epicardial AT as compared to subcutaneous AT,18 and it has been speculated that epicardial fat may play a paracrine role in diseases affecting the coronary arteries (atherothrombosis) and the myocardium (cardiac dysfunction).
Along this line, and given the association between leptin and proinflammatory markers, it could be expected that the level of expression of this hormone would also be higher in epicardial AT than in subcutaneous AT. In this respect, differences in leptin and adiponectin expression have been observed in different fat deposits, being more marked in subcutaneous AT than in visceral AT.16,17,31,32
This report shows that epicardial and subcutaneous AT produce different adiponectin and leptin concentrations, which are significantly higher in subcutaneous AT. This difference is mainly due to the influence of gender; when the results are analyzed according to the sex of the patients, the epicardial fat of the women was found to produce higher concentrations of adiponectin and leptin than that of the men (Figures 3A and 3B). Due to the fact that, in general, the level of adiponectin expression in AT is higher than that of leptin, the differences between epicardial and subcutaneous AT in terms of the expression of these hormones are more marked with respect to adiponectin than those observed with leptin. This finding could indicate that the adiponectin produced by AT plays a more relevant metabolic role than leptin.
In contrast to the results obtained by Iacobellis et al,19 who observed, in a group of 16 patients, that the epicardial AT of those with ischemic heart disease synthesized less adiponectin than that of the controls (aortic or mitral valve replacement), in our study, we found no significant differences between patients with ischemic heart disease (n=34) and those who underwent valve replacement (n=17) in terms of adiponectin expression. The lower levels of adiponectin and leptin expression in epicardial AT of the patients with ischemic heart disease did not reach statistical significance.
However, we can not rule out the possibility that the discrepancy encountered with respect to the data reported by Iacobellis et al be due to mechanisms of posttranscriptional regulation, which would explain the fact that no differences are observed in the levels of gene expression, and to differences in the characteristics of the patients, although not in the sample size, as we include a greater number of cases.
With respect to the results published by Baker et al,33 we also observed a lower level of expression of adiponectin and leptin mRNA in epicardial AT as compared to peripheral AT (subcutaneous, omental, and perimuscular).33 However, we consider the methodological validity of our approach to be greater since we employed both epicardial and subcutaneous AT from each patient, whereas Baker et al analyzed epicardial AT from patients who had undergone coronary artery surgery, comparing it with peripheral AT from other patients with no coronary artery disease or type 2 diabetes.33 Thus, they were unable to determine whether there were differences in the expression of these hormones in epicardial and peripheral fat from the same patient.
On the other hand, we can not rule out the possibility that a portion of the adipocytokine production be due to the presence of inflammatory cells in the AT, although adiponectin and leptin are produced mainly by the adipocytes, whereas macrophages are more specialized in the production of cytokines, such as tumor necrosis factor alpha or interleukin 6.34 Although 10% of the content of AT is compose of macrophages, the number of which is directly correlated with adiposity and the size of the adipocytes, there is no difference in the percentages of these inflammatory cells in subcutaneous and visceral AT. Thus, they can not be expected to influence the differences observed in our study.35
A number of factors could explain our findings with respect to the differences between men and women. The adiponectin concentrations may be lower in men due to the effect of androgens.36 Tsou et al37 described the influence of sex hormones on plasma plasma adiponectin levels, and the plasma leptin levels are also influenced by sex hormones.38 This relationship of adiponectin and leptin to androgens and estrogens may, in part, have determined our data, particularly the lower level of adiponectin activity in epicardial AT in men.
Our results may have certain clinical relevance. As we mentioned above, there is a relationship between the low adiponectin concentrations and factors directly implicated in atherothrombotic vascular disease, and a direct relationship between plasma adiponectin concentrations and the risk for a number of vascular diseases, in particular between decreased levels of this hormone and the risk of myocardial infarction.39 In this respect, the higher risk for vascular diseases of atherothrombotic etiology and, in particular, supposedly ischemic heart disease in men, could be related to the lower adiponectin production in epicardial AT, since, as we mentioned above, it could exert a direct paracrine effect on the coronary arteries, which would help to explain, in part, the differences in risk for atherothrombosis-related clinical conditions between men and women.
This possible pathophysiological explanation of the relationship between the metabolic activity of epicardial fat and the risk of coronary atherothrombosis is reinforced if we take into account the fact that the greatest proportion of epicardial fat is distributed in the epicardial coronary arteries.
CONCLUSIONS
In conclusion, we report for the first time that the production of leptin and adiponectin by epicardial AT is lower than that of subcutaneous AT. However, when the results are analyzed according to sex, a difference is observed in the expression of these hormones in epicardial AT, with a higher production in women than in men. The analysis of the ratio of adiponectin to leptin confirms that the difference in adiponectin expression in epicardial AT is related to sex, rather than to the presence of some type of inflammatory change, as could be observed after the comparison of the expression of these hormones in patients with ischemic heart disease and valve replacement.
ACKNOWLEDGEMENTS
We wish to thank Dr Otero, of the Biostatistics Department of the School of Medicine of the University of Santiago de Compostela, for his collaboration in the data analysis.
*These 2 authors contributed equally to the present report.
This study was financed by the Ministry of Health and Consumer Affairs, under the Program to Promote Research in Biomedicine and Health Sciences (grant no. PI040693).
Correspondence: Dr. J.R. González-Juanatey.
Laboratorio 1 Investigación y Docencia.
Unidad de Investigación en Cardiología Celular y Molecular (planta 0).
Hospital Clínico Santiago de Compostela.
Choupana, s/n. 15706 Santiago de Compostela. La Coruña. España.
E-mail: jose.ramon.gonzalez.juanatey@sergas.es
Received November 8, 2005.
Accepted for publication August 17, 2006.