Keywords
Functional ischemic mitral regurgitation is defined as a complication of coronary artery disease, rather than a chance association with mitral regurgitation of degenerative or some other etiology. It is a complex disease, the natural history of which is indicative of a poor prognosis. Grigioni et al1 reported a 5-year actuarial survival of 38%±5%, with a significant impact on quality of life. The rate of survival was significantly lower (29%±9%) among patients with an effective regurgitant orifice area of 20 mm² or more.
The complex functional anatomical and pathophysiological mechanisms that cause ischemic mitral regurgitation are not entirely clear. Left ventricular remodeling owing to severe myocardial infarction plays an important role in its development, although local involvement of the papillary muscles is also a factor. In contrast to its role in other types of mitral insufficiency, annular dilation is not the direct cause of regurgitation, but the consequence of the different mechanisms leading to a lack of valve coaptation.
It is not always easy to establish the etiology of functional mitral regurgitation in coronary artery disease because the latter presents with degenerative mitral regurgitation in a considerable proportion of patients. Valve lesions due to Barlow disease can be identified by the typical redundant, billowing leaflets with elongated chordae tendineae. In contrast, the coexistence of an isolated degenerative lesion in the posterior leaflet makes the etiological diagnosis of mitral regurgitation more difficult, both on echocardiographic examination and on direct intraoperative inspection. The ischemic origin of the lesion is evident when the posterior papillary muscle appears to be elongated and presents a pearly aspect or partial rupture.
The therapeutic approach to this disease is the subject of controversy since the results of mitral valve surgery continue to be unsatisfactory. Functional ischemic mitral regurgitation is currently one of the most widely investigated medical conditions at the international level. In recent years, a number of new aspects of its anatomy and pathophysiology have been discovered, and novel surgical techniques are helping to improve the outcome of the correction of this disease.
The purpose of this editorial is to compile the most relevant and valuable scientific information encompassed in the knowledge of the different underlying mechanisms of functional ischemic mitral regurgitation, as well as the new surgical techniques.
FUNCTIONAL ANATOMY
In patients with nonischemic mitral regurgitation, the abnormal valve structure leads to functional deterioration of left ventricle because of dilation and, eventually, to ventricular dysfunction. In contrast, the ventricular dysfunction in coronary artery disease generates a series of changes in left ventricular geometry and in the various components of the mitral valve (papillary muscles, chordae, leaflets, and annulus), resulting in the complex functional anatomy of ischemic mitral regurgitation. Coronary heart disease can lead to left ventricular dysfunction, with changes in the papillary muscles, rupture or detachment of the chordae tendineae, restricted valve motion, annular dilation, and, consequently, mitral regurgitation. The progressive increase in mitral regurgitation results in an increase in ventricular dilation and wall stress, thus establishing a vicious circle, with progressive deterioration of ventricular function and aggravation of mitral regurgitation.2-4
Transthoracic and transesophageal echocardiography have contributed substantially to the understanding of the mechanisms of ischemic mitral regurgitation.5,6 As it depends on left ventricular function, mitral regurgitation acts as a functional failure, the severity of which varies from one moment to another. Thus, it is important to carefully analyze the valve anatomy, which remains constant, rather than its function, which changes over time. Craig Miller's research team at Stanford University has made a major contribution to the understanding of this complex valve disease through a number of experimental studies in sheep, in which they induced myocardial ischemia for subsequent testing of different annuloplasty devices.7-9 However, these studies in animals with acute myocardial ischemia do not faithfully reproduce the anatomical and pathophysiological features of the clinical picture in humans and, thus, their value is limited.
Basically, mitral valve anatomy exhibits restricted leaflet motion and annular dilation, resulting in inadequate coaptation. For this reason, it has been included in the Carpentier classification of mitral insufficiency as type IIIb.10 Since 1983, this classical classification,10 which has not undergone subsequent revision, groups the different types of mitral insufficiency according to the mobility of the valve leaflets. Type I includes mitral insufficiency with normal valve leaflet mobility, in which the valvular regurgitation is mainly produced by the lack of leaflet coaptation (annular dilation) or perforation (endocarditis or trauma). Type II is comprised of insufficiency with increased valve mobility due to chordal elongation or rupture (degenerative disease). Finally, type III includes mitral insufficiency in which valve motion is restricted (type IIIa: rheumatic valve disease; type IIIb: coronary artery disease). However, recent findings associated with coronary artery disease have limited the usefulness of this classification. Segmental ischemia of left ventricle, akinetic or dyskinetic, and of the papillary muscles --in particular, the posterior papillary muscle--provokes a restriction of valve motion (type IIIb). This is due to the increase in the distance between the mitral annular plane and the papillary muscle base. The excessive tension on the chordae tendineae impedes the normal coaptation of the valve leaflets, a finding that is most evident in the region of the posterior commissure and the region posterior to the septal leaflet. Moreover, mitral insufficiency can also present as a simple functional failure secondary to annular dilation (type I) or, less frequently, with valve prolapse (type II), as recently observed by Jouan et al11 in one third of their patients. Prolapse usually occurs in the presence of necrosis of the papillary muscle or one of its heads into which marginal chordae tendineae are inserted. This papillary muscle ischemia can provoke its partial rupture or its dysfunction, events that contribute to valve prolapse. Myocardial ischemia usually affects the posterior papillary muscle due to its precarious irrigation, although the anterior papillary muscle may also be involved (in 9% of the cases),11 despite the fact that its vascularization is better ensured by the left anterior descending and diagonal arteries.
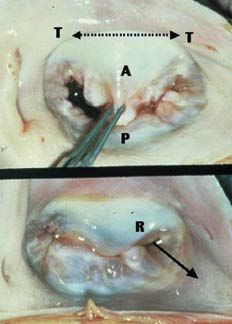
The magnetic resonance imaging study performed in China by Yu et al12 demonstrated that the mechanism that produces mitral insufficiency is very complex and involves the participation of multiple vector forces originated by the increase in the anteroposterior diameter of the annulus, the abnormal posteroinferior tension on the leaflets, and increases in the distance between the papillary muscles and in the distance between the annulus and the base of these muscles. Using a computer program involving three-dimensional echocardiography, Ahmad et al13 made some highly interesting observations concerning annular geometry and valve motion in ischemic mitral regurgitation. The annular perimeter was significantly greater than that of the control group (10.7 cm vs 8.6 cm) and there was an increase in the intertrigonal distance (2.8 cm vs 2.1 cm), resulting in a significant increase in the mitral orifice area. In addition, the patients presented an overall restriction of mitral annulus motion, especially in the posterior region (5.4 cm vs 8.7 cm). This elegant study demonstrated that the increases in both the anteroposterior diameter of the annulus and in the intertrigonal distance play an important role in the underlying mechanism of mitral insufficiency. These findings contradict the traditional concept, introduced by Carpentier,10 that the intertrigonal zone of the mitral annulus experiences no dilation whatsoever as it is connected to the annulus fibrosus of the aortic valve, and that only the zone of the annulus corresponding to the commissures and the posterior leaflet increases in size. In functional ischemic mitral regurgitation, all the segments of the mitral annulus, including the intertrigonal region (Figure), are dilated, especially during the end-diastolic phase. Hueb et al14 demonstrated that, in these patients, the intertrigonal distance increases in length by 33%. This finding has been confirmed by other authors,15,16 and appears to be even more evident in the study of the Cleveland Clinic group.13 The mitral annulus, which normally has the shape of a saddle, experiences spatial motion throughout the cardiac cycle. Three-dimensional echocardiographic analysis demonstrated a significant restriction of this motion in mitral insufficiency of ischemic origin. In these patients, it has been shown that, between the end-systolic and end-diastolic phases, the region of the posterior leaflet and posterior commissure undergoes changes in the annular configuration and that the spatial annular motion is significantly limited.13,17,18
Figure. Functional ischemic mitral regurgitation. A indicates anterior leaflet; P, posterior leaflet; T, trigone; R, mitral regurgitation in the posterior commissural region showing the direction of the jet (continuous arrow). The discontinuous arrow indicates the increased intertrigonal distance.
As the underlying mechanisms of mitral regurgitation progress, the area of contact between the anterior and posterior leaflets decreases. The echocardiographic study provides valuable information regarding the changes in the area of valve contact. The papillary muscles gradually undergo posterolateral and apical displacement, which increases the tensile stresses to which the leaflets are subjected, limiting their motion towards the normal valve plane. The distance between the valve plane and the leaflet coaptation point constitutes an excellent predictive factor for the results of repair. The displacement of this coaptation point is directly proportional to the tensile stresses, giving us a clear idea of the relationship between the papillary muscles and the insertion of the leaflets into the annulus. Calafiore et al19,20 found that when the coaptation point was at a distance of 10 mm or less from the normal annular plane, the long-term postoperative results of mitral repair were excellent. On the other hand, if the distance was greater than 10 mm, indicating a considerable tension on the subvalvular apparatus, which impedes adequate valve coaptation, the results of valve reconstruction were usually unsatisfactory, with a high percentage of cases of residual mitral regurgitation. For this reason, in these patients, the replacement of the mitral valve is recommended. Thus, functional ischemic mitral regurgitation is caused by interrelated changes in ventricular geometry that not only affect leaflet coaptation, but distort considerably the location and function of the different elements that constitute the complex mitral subvalvular apparatus.
RECONSTRUCTION OR REPLACEMENT OF THE MITRAL VALVE
The natural history of this disease suggests that surgery, at least in the case of severe functional ischemic mitral regurgitation (4+), is the best option for improving survival. However, there is no agreement concerning the benefits of surgery in patients with mild (2+) or moderate (3+) regurgitation. Until recently, it was recommended that complete coronary revascularization alone be performed, without mitral valve surgery, since the latter had a negative effect on the surgical results. In contrast, other authors have reported that mitral surgery in coronary patients significantly improves the postoperative results. Several clinical studies have reported an elevated medium-term mortality following prosthetic replacement in ischemic mitral regurgitation, with the death of nearly half of the patients within the first 5 postoperative years. A few years ago, Cohn et al21 argued that mitral valve replacement was preferable since the 5-year actuarial survival rate was significantly higher (91%) than that associated with mitral repair (56%). At the present time, the improved knowledge of the underlying mechanisms of ischemic mitral regurgitation has radically changed this concept. In 2004, Calafiore et al20 reported a better 5-year survival rate in patients who underwent mitral valve repair than in those with prosthetic replacement (75.6% vs 66%). This finding has been confirmed by other authors, including Gillinov et al22 and Grossi et al.23 On the other hand, the association of moderate mitral regurgitation with coronary disease indicates a worse prognosis in patients undergoing myocardial revascularization. In Toronto, Paparella et al24 demonstrated that the 10-year survival of patients with coronary disease and moderate ischemic mitral regurgitation who undergo coronary bypass alone was lower than that of a group of patients who did not have mitral insufficiency (53% vs 75%). In patients with moderate or severe left ventricular dysfunction, failure to repair the mitral valve in cases of mitral regurgitation impedes ventricular restoration, as reported by Isomura et al,25 a circumstance that significantly affects medium and long-term survival. Thus, in view of the clear evidence of the superiority of repair techniques in terms of survival and the reduction of postoperative complications, mitral valve repair is always recommended in cases of moderate (3+) or severe (4+) functional ischemic mitral regurgitation.22,23,26-29
TYPE OF MITRAL REPAIR
Ischemic mitral regurgitation is difficult to correct because of the diverse mechanisms, some of them poorly understood, involved in this complex disease. Mitral annuloplasty only partially resolves the mitral ischemic lesion, favoring the coaptation of the anterior and posterior leaflets by reducing the anteroposterior diameter of the mitral orifice. However, it does not correct the rest of the mechanisms that produce the valve insufficiency and, thus, stable valve competence is not ensured. Several factors contribute to recurrent mitral regurgitation, including the preoperative degree of left ventricular function, the severity of regurgitation, the location of the valve coaptation point with respect to the annular plane and the direction of the regurgitant jet as viewed in the echocardiographic study. The presence of a central jet indicates that the mobility of both leaflets is restricted by excessive tension on the chordae tendineae, which makes it difficult to achieve adequate coaptation with no residual regurgitation. The presence of an eccentric jet, the most common finding, indicates the presence of a localized restriction in the region of the posterior commissure and the most posterior portion of the posterior leaflet (P3); this anatomical variant is that most easily corrected by surgery (Figure).
The technique of choice for mitral annuloplasty remains a cause for debate since a few months after surgery (6 months on average), mitral regurgitation (>2+) recurs in 17% to 29% of the patients.30-32 It has been known for years that the durability of annuloplasty involving suturing (de Vega mitral annuloplasty) was limited,27 with early recurrence of mitral regurgitation occurring in the majority of cases. McGee et al,33 of the Cleveland Clinic, have reported their extensive experience in 585 patients with ischemic mitral regurgitation who underwent annuloplasty using the Cosgrove-Edwards® partial flexible ring, the Carpentier Classic® rigid ring or the Peri-Guard® bovine pericardium ring. These authors demonstrated that 28% of the surgical patients presented moderate (3+) or severe (4+) mitral regurgitation within the first 6 postoperative months. The lesion recurred more frequently after annuloplasty involving the biological pericardium ring than when prosthetic rings were employed (66% vs 25%),33 although the type of annuloplasty did not influence survival. After those first 6 months, there was no evidence of an increase in the rate of recurrence of mitral regurgitation. An asymmetric ring (the Carpentier-McCarthy-Adams ETlogix® ring) has been introduced that, aside from reducing the anteroposterior diameter of the mitral orifice in order to ensure leaflet coaptation, specifically corrects the posterior region of the valve (posterior commissure and posterior zone of the septal leaflet). Thus, this new prosthetic ring may improve the durability of mitral repair. There still remains a certain controversy with respect to the advisability of utilizing smaller rings (two sizes smaller than that indicated by the measurer) to achieve greater leaflet coaptation, a strategy that has produced highly satisfactory results,19,34 although these data have not been confirmed by some authors.29,33 Bax et al35 employed this restrictive annuloplasty involving the implantation of a rigid ring (Physio ring®) two sizes too small with excellent results, including the absence of residual mitral regurgitation and a clear improvement in ventricular remodeling. The latter authors consider that this restrictive annuloplasty achieves valve competence through optimal coaptation of the anterior and posterior leaflets (mean 0.8±0.2 cm). The recent finding that the intertrigonal distance can dilate significantly partly explains the satisfactory results obtained by these authors with restrictive annuloplasty (using a prosthetic ring two sizes smaller than that indicated by the measurer). The maneuver for measuring the size of the prosthetic ring is based on the intertrigonal distance which, according to Carpentier's classical concept, is a reliable measurement since its length, representing one third of the normal annular circumference, is constant. This erroneous measurement system, which takes the dilated annular region between the two trigones as a reference, induces the surgeon to employ a larger ring than that necessary for the annuloplasty to achieve the objective of ensuring adequate leaflet coaptation. This anatomical finding also explains the fact that annuloplasty involving an incomplete (U-shaped) ring frequently does not correct mitral regurgitation in patients in whom the intertrigonal distance has not been properl y dealt with. This circumstance has also been suggested very recently by Gómez-Durán.36
A new annuloplasty system has been developed (Coapsys Annuloplasty System, Myocor®)37 that consists of the implantation of artificial chordae made of polytetrafluoroethylene (PTFE) that are introduced through the left ventricular cavity and secured to the epicardium by means of synthetic buttons. These PTFE chordae diminish the left ventricular cavity significantly, as they reduce the distance between the mitral annulus and the base of the papillary muscles, as well as the anteroposterior diameter of the mitral orifice and the distance between the papillary muscles. With these artificial chordae, the effect of the abnormal tension on the leaflets is considerably reduced and mitral regurgitation is corrected. Although experience with it is still limited, an initial report, involving 22 patients into whom these intraventricular chordae had been implanted with highly satisfactory results, was presented at the last annual meeting of the European Association for Cardio-thoracic Surgery (Leipzig, 2004). Several groups in the United States have undertaken a randomized clinical trial (RESTOR-MV) for the Food and Drug Administration, in which they employ this annuloplasty system, taking into account the regression of ventricular remodeling as a mechanism that induces chronic ischemic mitral regurgitation.
COMMENTS
Functional ischemic mitral regurgitation is a dynamic process that is directly related to ventricular remodeling. Thus, the degree of severity varies according to left ventricular contractility. The role of echocardiography in decision making concerning this functional regurgitation is fundamental. The underlying mechanisms are highly diverse and still not well understood. They include the degree of ischemic damage to the papillary muscles, the tension on the leaflets, especially on the posterior leaflet and posterior commissure, the level of the point of valvular contact (kissing edge), occasionally valve prolapse, and the consequent annular dilation. The complexity of this valve lesion has refuted a classical concept that had remained unchallenged for 30 years: that the mitral annular region between the 2 trigones was incapable of dilating, as occurs with the rest of the mitral annulus fibrosus. It has been demonstrated that, in patients with functional ischemic mitral regurgitation, this annular zone can dilate by up to 33%.
On the other hand, Carpentier's classification of mitral insufficiency,10 internationally recognized for its diagnostic utility, has been shown to be unsuitable for the classification of functional ischemic mitral regurgitation since this entity can be grouped with type I (annular dilation), II (valve prolapse), or IIIb (restricted leaflet motion). Once the underlying mechanisms of mitral regurgitation and its intimate relationship to ventricular remodeling are fully understood, this classification may have to be modified to include a new group (type IV: variable valve mobility). This would make it possible to properly classify functional ischemic mitral insufficiency according to its different anatomical and functional aspects.
This lesion, misunderstood for years, has been treated surgically by valve replacement or repair, generally with unsatisfactory results. At the present time, with the better understanding of its complex anatomy and pathophysiology, the results of surgery are improving significantly. The experience of a number of groups demonstrates that the outcome of mitral repair is significantly better than that of prosthetic replacement in terms of survival and postoperative complications.19,22,23 However, it is necessary to continue to investigate new methods of valve reconstruction that enable the reversal, at least in part, of ventricular remodeling and mitigate the consequences of it. In this respect, new annuloplasty techniques (for example, the ETlogix® asymmetric ring) and devices designed to address the anatomy of the subvalvular apparatus and ventricular dilation (such as the Myocor® artificial chordae) are being tested, with satisfactory initial results.
Advances in diagnostic imaging techniques and increased experience in surgical treatment of this disease will continue to open doors to the understanding of functional ischemic mitral regurgitation, a circumstance that will improve the natural history of this still enigmatic valve disease.
Correspondence: Dr. J. M. Revuelta.
Servicio de Cirugía Cardiovascular. Hospital Universitario Marqués de Valdecilla.
Avda. Valdecilla, s/n. 39008 Santander. Cantabria. España
E-mail: revuelta@humv.es