Epicardial adipose tissue has been associated with several obesity-related parameters and with insulin resistance. Echocardiographic assessment of this tissue is an easy and reliable marker of cardiometabolic risk. However, there are insufficient studies on the relationship between epicardial fat and insulin resistance during the postmenopausal period, when cardiovascular risk increases in women. The objective of this study was to examine the association between epicardial adipose tissue and visceral adipose tissue, waist circumference, body mass index, and insulin resistance in postmenopausal women.
MethodsA cross sectional study was conducted in 34 postmenopausal women with and without metabolic syndrome. All participants underwent a transthoracic echocardiogram and body composition analysis.
ResultsA positive correlation was observed between epicardial fat and visceral adipose tissue, body mass index, and waist circumference. The values of these correlations of epicardial fat thickness overlying the aorta-right ventricle were r = 0.505 (P < .003), r = 0.545 (P < .001), and r = 0.515 (P < .003), respectively. Epicardial adipose tissue was higher in postmenopausal women with metabolic syndrome than in those without this syndrome (mean [standard deviation], 544.2 [122.9] vs 363.6 [162.3] mm2; P = .03).
ConclusionsEpicardial fat thickness measured by echocardiography was associated with visceral adipose tissue and other obesity parameters. Epicardial adipose tissue was higher in postmenopausal women with metabolic syndrome. Therefore, echocardiographic assessment of epicardial fat may be a simple and reliable marker of cardiovascular risk in postmenopausal women.
Keywords
Cardiovascular risk and the metabolic syndrome (MS) increase after menopause.1 Changes in sexual hormone concentrations that occur during this period affect insulin resistance and the distribution of visceral and subcutaneous adipose tissue.1,2
For many years, adipose tissue was considered to be stored energy. However, in 1994 this concept was revised when adipose tissue was discovered to secrete the hormone leptin; since then, this tissue has been recognized to function as an endocrine organ that produces hormones or adipokines.3,4 Obesity is characterized by adipocyte hypertrophy and hyperplasia and by changes in adipokine secretion, which contribute to increased insulin resistance and inflammation.3 Visceral adipose tissue (VAT) is the tissue covering the internal organs and its increase is related to a cardiometabolic risk profile.4,5
Epicardial adipose tissue (EAT) has the same embryonic origin as intraabdominal adipose tissue.6 This type of tissue is localized on the myocardium, in the atrioventricular and interventricular sulci, extends to the apex, and surrounds the coronary arteries.7 This fatty tissue is highly active and produces numerous adipokines, including proinflammatory and proatherogenic cytokines, such as tumor necrosis factor-alpha, type-1 plasminogen activator inhibitor, interleukin-6, visfatin, leptin, omentin, and angiotensin.8 Epicardial fat also serves as an energy source for the myocardium and protects it from fatty acid toxicity.8,9
Echocardiographic assessment of EAT is directly associated with VAT accumulation, even more so than some anthropometric variables, such as waist circumference (WC).10
The aim of this study was to analyze the association among EAT and other obesity-related parameters, such as VAT, WC, body mass index (BMI), and insulin resistance.
METHODSA cross-sectional, comparative study was performed in 34 postmenopausal women aged 50 to 65 years who consecutively attended the Endocrine Diseases Medical Research Unit of the Hospital de Especialidades del Centro Médico Nacional IMSS. Diagnosis of menopause was confirmed by low serum estradiol concentrations and increased follitropin values. None of the participants was receiving hormone replacement therapy. The participants were divided into 2 study groups: patients diagnosed with MS and women without MS. Diagnosis of MS was based on the criteria for clinical practice of the International Diabetes Federation.11,12 These criteria consist of central obesity (defined as WC ≥ 80 cm) in addition to 2 of the following components: triglyceride levels equal to or greater than 150 mg/dL, reduction of high-density lipoprotein cholesterol less than 50 mg/dL, systolic blood pressure equal to or greater than 130mmHg or diastolic blood pressure equal to or greater than 85mmHg, and/or fasting glucose equal to or greater than 100mg/dL.
Women were excluded if they had an established diagnosis of diabetes mellitus, renal or liver failure, chronic infections, endocrine or blood disorders, or a history of cardiovascular disease or thrombosis. Also excluded were women who were receiving anticoagulant therapy. The study protocol was approved by the Ethics Committee of the Mexican Institute of Social Security. Participants were informed about the study and provided their written informed consent.
Clinical EvaluationA clinical history and anthropometric measurements were taken from all patients. The patients were weighed and measured without shoes and with light clothing on a Bame weight and height scale. Systolic and diastolic blood pressure were determined with an aneroid baumanometer. Hip circumference and WC were measured. Body mass index was calculated as weight in kilograms divided by height in meters squared.
Body Composition AnalysisBody composition was assessed with a 353 ioi JAWON body composition analyzer. Bioelectrical impedance was analyzed in the morning after a 12-hour fast and adequate hydration. Bioelectric impedance was measured with the patient standing up and wearing light clothing, without shoes. The analyzer measured weight with an accuracy of within 0.1kg, as well as body impedance (in ohms), with calculation of the VAT value and the percentage of total body fat.
Biochemical AnalysisVenous blood samples were drawn from the antecubital veins between 8.00 and 9.00, after a fast equal to or greater than 12 hours. The samples were collected in tubes without coagulant and were centrifuged at 3500 rpm for 20 minutes to separate the serum and prepare 500 μL-aliquots, which were kept frozen at –70°C until assayed. Glucose, high-density lipoprotein cholesterol, and triglyceride levels were determined in serum through the semiautomatic chemical analyzer Ekem Control Lab. Insulin was measured by a solid-phase radioimmunoassay (Millipore, Billerica; Mississippi, United States); the sensitivity of this assay was 2 μU/mL and the intra- and interanalytic coefficient of variation was 4.0% and 8.6%, respectively. Insulin resistance was evaluated through homeostasis model assessment (HOMA) according to the method of Matthews et al13:
HOMA-IR = insulin (μU/mL) × fasting glucose (mmol/L) / 22.5
Echocardiographic Evaluation of Epicardial Adipose TissueTo evaluate cardiac structure, all participants underwent transthoracic M-mode, 2-dimensional and Doppler echocardiography (Phillips IE33 echocardiography, version 5.2.0.289). Standard methodology was used to obtain the images, with the patient placed in the left lateral decubitus position and display of images on the echocardiographic system.14 Images were taken in parasternal long-axis and 4-chamber views. Epicardial fat was identified as hypoechoic tissue surrounding the heart immediately over the myocardium or below the visceral layer of the pericardium.15 This tissue becomes compressed in diastole and becomes thicker in systole.15,16
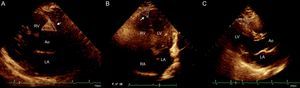
Four sites of epicardial fat deposits were selected, using the depth of the field to improve 2-dimensional visualization. Two of these sites were visualized in the parasternal long-axis view: the sulcus between the aortic root and the right ventricle, which forms a triangle with the external base and the cusp where the aorta connects with the right ventricle, and the site between the right ventricle and the apical portion (right ventricular sulcus-apical portion). In the 4-chamber view, the left sulcus was measured; this sulcus is situated between the atrium and the left ventricle (left auriculoventricular sulcus). In the same view, epicardial fat thickness was measured at the apex, which was observed with the morphology of the inverted triangle with the base above and the tip of the triangle below, at the connection between the apical regions of both ventricles. The linear measure was used, taking 2 main axes, vertical and horizontal; a basic principle was that sulci full of fatty tissue appear in the shape of a triangle and, consequently, the area of these “fatty triangles” was obtained on the basis of these 2 measurements (Figure 1). In addition, adipose tissue was measured on the free wall of the right ventricle by using parasternal long-axis and short-axis views, as previously described.17,18 All measurements were made by the same echocardiographer.
Echocardiographic evaluation of epicardial fat. A: Epicardial fat in the right aortoventricular sulcus, between the aortic root and the right ventricle. B: Epicardial fat in the apical sulcus located between the apexes of the 2 ventricles. C: Epicardial fat in the right ventricular sulcus-apical portion. In all cases, measurements were taken of the base and height and planimetry was used to signal the site of interest. RA, right atrium; LA, left atrium; Ao, aorta; RV, right ventricle; LV, left ventricle.
Nonparametrical tests were used due to the distribution of the variables. The correlation among variables was identified by Spearman's test and differences among groups were identified by the Mann-Whitney U test. All statistical analyses was performed with the statistical package, SPSS v.14.
A sample of 31 participants was needed to obtain a statistical power of 90% and a correlation of at least 0.50,19 with a P-value p < .05.
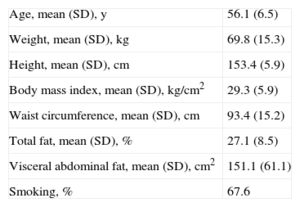
RESULTSA total of 34 women were evaluated, with a mean age of 56.1 years (SD, 6.5) BMI ranging widely from 22 to 40 (Table 1). The mean body fat value was within the upper normal range (27.1% [SD, 8.5%]); however, abdominal fat values were higher (mean, 151.1 [SD, 61.1] [interval, 32-194] cm2).
General Characteristics of the Participants
| Age, mean (SD), y | 56.1 (6.5) |
| Weight, mean (SD), kg | 69.8 (15.3) |
| Height, mean (SD), cm | 153.4 (5.9) |
| Body mass index, mean (SD), kg/cm2 | 29.3 (5.9) |
| Waist circumference, mean (SD), cm | 93.4 (15.2) |
| Total fat, mean (SD), % | 27.1 (8.5) |
| Visceral abdominal fat, mean (SD), cm2 | 151.1 (61.1) |
| Smoking, % | 67.6 |
SD, standard deviation. Unless otherwise indicated, values are expressed as mean (standardard deviation).
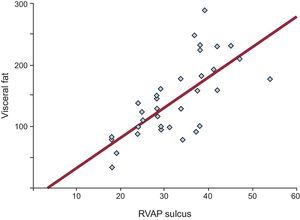
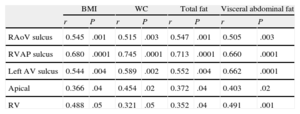
All linear measurements of EAT showed a positive and significant correlation with the distinct obesity parameters (Table 2). Adipose tissue thickness over the right ventricle was correlated with BMI (r = 0.488; P = .05), WC (r = 0.321; P = .05), total body fat (r = 0.352; P = .04) and VAT (r = 0.491; P = .001). The highest correlation between VAT and EAT was found in the fatty area of the right ventricular sulcus-apical portion (r = 0.680; P < .0001) (Figure 2).
Association Between Obesity Parameters and Epicardial Fat Measurements
| BMI | WC | Total fat | Visceral abdominal fat | |||||
| r | P | r | P | r | P | r | P | |
| RAoV sulcus | 0.545 | .001 | 0.515 | .003 | 0.547 | .001 | 0.505 | .003 |
| RVAP sulcus | 0.680 | .0001 | 0.745 | .0001 | 0.713 | .0001 | 0.660 | .0001 |
| Left AV sulcus | 0.544 | .004 | 0.589 | .002 | 0.552 | .004 | 0.662 | .0001 |
| Apical | 0.366 | .04 | 0.454 | .02 | 0.372 | .04 | 0.403 | .02 |
| RV | 0.488 | .05 | 0.321 | .05 | 0.352 | .04 | 0.491 | .001 |
BMI, body mass index; Left AV, left auriculoventricular; RAoV, right aortoventricular; RV, thickness in the free wall of the right ventricle RVAP, right ventricle-apical portion; WC, waist circumference.
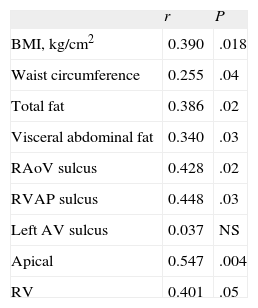
Insulin resistance was significantly correlated with adiposity parameters and with all measurements of EAT, except in the left auriculoventricular sulcus (Table 3). The value of this association with insulin resistance was r = 0.428 in the right aortoventricular sulcus (P = 0.02) and was r = 0.401 with right ventricular thickness (P < .05).
Correlations of Insulin Resistance (Evaluated by Homeostasis Model Assessment) With Epicardial Fat and Obesity Parameters
| r | P | |
| BMI, kg/cm2 | 0.390 | .018 |
| Waist circumference | 0.255 | .04 |
| Total fat | 0.386 | .02 |
| Visceral abdominal fat | 0.340 | .03 |
| RAoV sulcus | 0.428 | .02 |
| RVAP sulcus | 0.448 | .03 |
| Left AV sulcus | 0.037 | NS |
| Apical | 0.547 | .004 |
| RV | 0.401 | .05 |
BMI, body mass index; left AV, left auriculoventricular; RAoV, right aortoventricular; RV, thickness in the free wall of the right ventricle; RVAP, Right ventricle-apical portion.
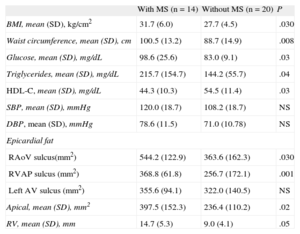
Of the study participants, 41% had MS. These participants consequently had higher BMI and glucose and triglyceride concentrations and lower high-density lipoprotein concentrations than women without MS (Table 4). Women with MS also had a greater quantity of EAT; this difference between women with and without MS was 544.2 (SD, 122.9) vs 363.6 (SD,162.3) mm2 for right the aortoventricular sulcus (P = .03) and 14.7 (SD,5.3) vs 9.0 (SD,4.1) mm for right ventricular thickness (P < .05), respectively.
Epicardial Fat in Participants With and Without Metabolic Syndrome
| With MS (n = 14) | Without MS (n = 20) | P | |
| BMI, mean (SD), kg/cm2 | 31.7 (6.0) | 27.7 (4.5) | .030 |
| Waist circumference, mean (SD), cm | 100.5 (13.2) | 88.7 (14.9) | .008 |
| Glucose, mean (SD), mg/dL | 98.6 (25.6) | 83.0 (9.1) | .03 |
| Triglycerides, mean (SD), mg/dL | 215.7 (154.7) | 144.2 (55.7) | .04 |
| HDL-C, mean (SD), mg/dL | 44.3 (10.3) | 54.5 (11.4) | .03 |
| SBP, mean (SD), mmHg | 120.0 (18.7) | 108.2 (18.7) | NS |
| DBP, mean (SD), mmHg | 78.6 (11.5) | 71.0 (10.78) | NS |
| Epicardial fat | |||
| RAoV sulcus(mm2) | 544.2 (122.9) | 363.6 (162.3) | .030 |
| RVAP sulcus (mm2) | 368.8 (61.8) | 256.7 (172.1) | .001 |
| Left AV sulcus (mm2) | 355.6 (94.1) | 322.0 (140.5) | NS |
| Apical, mean (SD), mm2 | 397.5 (152.3) | 236.4 (110.2) | .02 |
| RV, mean (SD), mm | 14.7 (5.3) | 9.0 (4.1) | .05 |
BMI, body mass index; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; left AV, left auriculoventricular; MS, metabolic syndrome; NS, nonsignificant; RAoV, right aortoventricular; RV, thickness in the free wall of the right ventricle; RVAP, right ventricle-apical portion; SBP, systolic blood pressure.
This study demonstrates an association between the quantity of EAT evaluated by echocardiography and VAT, BMI, WC, and the quantity of body fat. Epicardial adipose tissue is able to modulate cardiac physiology locally and may play a causal role in atherosclerosis and cardiovascular events.7,20,21 Epicardial fat is metabolically active and adjacent to the myocardium and thus, under pathological conditions, this fat can directly affect the coronary arteries and the heart through secretion of proinflammatory substances.20,22 Several studies have shown that EAT can play a role in the development of coronary disease, although the exact mechanisms remain to be elucidated.23
Epicardial fat has a closer association with VAT than with total body fat and epicardial fat thickness may reflect the quantity of VAT.10 This study found a high association between these 2 types of adipose tissue; although WC is an easy-to-measure marker of VAT, it has low specificity for visceral adiposity. Consequently, several studies have indicated that echocardiographic measurement of epicardial fat can provide a more specific measurement of intraabdominal visceral fat, thus avoiding possible confusion due to the increase in subcutaneous adipose tissue in the abdomen.7,17
Another interesting finding of this study is the close association between EAT and insulin resistance. The data in the present study are similar to those in 2 previously published studies that also found a direct association between insulin resistance and EAT in both men and women.17–24 In the present study, this direct association between insulin resistance and EAT was observed in postmenopausal women. Equally, various clinical studies have demonstrated an association between increased EAT and cardiovascular risk factors. Two studies with an open population and participants of both sexes reported that EAT is thicker in persons with MS.25,26 These results are similar to those of the present study, which found that postmenopausal women with MS also had a higher quantity of EAT than women without cardiometabolic factors. In contrast with other studies that included both men and women, the present study focussed on women, not only because there are important differences in the distribution of risk factors between the sexes but also because there are also some differences in EAT. 27 The quantities of adiponectin and leptin are greater in the EAT of women than in that of men, and these differences could be due to the effect of sexual hormones.28
Despite the potential importance of EAT measurement, consensus is lacking on how it should be measured; some groups, such as that of Iacobellis et al,18 have proposed that echocardiography is a simple method and has a very high correlation with magnetic resonance measurement (r = 0.901), with the advantage of its low cost, good reproducibility, and noninvasiveness; these properties allow echocardiography to be considered as an easy method for evaluating cardiometabolic risk factors and MS.18
In the present study, visceral and body fat were measured by bioelectrical impedance, which could be considered a limitation, since computed tomography evaluation is believed to be more precise. However, bioelectric impedance is inexpensive and easy to perform and is also safer than computed tomography because it avoids radiation exposure.29 Bioelectric impedance has a close correlation (r = 0.905) with computed tomography in the evaluation of VAT.29 In addition, although the sample in the present study consisted of 34 participants, the statistical analysis confirmed that this number was sufficient to achieve a high statistical power and to detect the association between EAT and other adiposity parameters.
The main strength of this study is that it was conducted in postmenopausal women; to our knowledge, this is the first study to use echocardiography to measure EAT at this stage of women's life cycle. Study of postmenopausal women is important because, during this stage, some risk factors, such as insulin resistance and abdominal fat increase, which, together with other changes, such as those occurring in lipid profile, increase cardiovascular risk.30
All the sites where EAT was measured showed a correlation with abdominal and body fat, but the left auriculoventricular sulcus showed the lowest association with insulin resistance and MS. The most accessible and reproducible site for measurement is probably the right aortoventricular sulcus, since the borders in this sulcus are well delimited. In contrast, measurement at the right ventricular sulcus-apical portion may be influenced by the echocardiographic window; consequently, standardization of the technique is advisable. In addition to linear EAT measurements, this study also analyzed a simple method to evaluate the fatty area in the grooves, the main areas of the heart with deposition of adipose tissue.4,6 Measurement of the area of these grooves showed a closer association with BMI, total body fat, visceral body fat, and insulin resistance.
CONCLUSIONSThis study showed a close association between EAT measured by echocardiography and abdominal fat and other adiposity parameters in postmenopausal women. The quantity of EAT was also related to the degree of insulin resistance and the presence of MS. Consequently, echocardiographic measurement of EAT could be highly useful to identify women at high cardiovascular risk during menopause.
CONFLICT Of INTERESTNone declared.
The authors Lourdes Basurto Acevedo and Arturo Zárate Treviño thank the Sistema Nacional de Investigadores-CONACyT for their research grant.