Several interventions can improve low-density lipoprotein cholesterol (LDL-C) control. Our main objective was to evaluate the efficacy of a combined intervention to improve LDL-C control in patients with hypercholesterolemia. The study also assessed the efficacy of the intervention in improving adherence (pharmacological, diet, and exercise).
MethodsA multicenter, parallel group, randomized clinical trial (primary care) was conducted in 358 adults diagnosed with hypercholesterolemia, whether receiving prior drug therapy or not. We compared 178 participants who received the combined intervention (written material, self-completed registration cards, and messages to mobile telephones) with 178 controls. The main outcome variable was the proportion of participants with adequate LDL-C control (target levels of the European guidelines on dyslipidemia and cardiovascular risk) at 24 months.
ResultsAt 24 months, the mean reduction in LDL-C was significantly higher in the intervention group (23.8mg/dL [95%CI, 17.5-30.1]) than in the control group (14.6mg/dL [95%CI, 8.9-20.4]; P=.034). The mean LDL-C decrease was 13.1%±28.6%. At 1 year, the proportion of participants with adequate control was significantly higher in the intervention group than in the control group (43.7% vs 30.1%; P=.011; RR, 1.46). Adherence was significantly higher in the intervention group, both to drug therapy (77.2% vs 64.1%; P=.029) and exercise (64.9% vs 35.8; P<.001), but not to diet.
ConclusionsThe combined intervention significantly reduced LDL-C (by more than 13% at 2 years) and improved the degree of LDL-C control in patients with hypercholesterolemia at 1 year.
Keywords
The prevalence of hypercholesterolemia in Spain ranges from 20% to 50%, depending on the plasma cholesterol concentrations considered.1,2
For better cholesterol control in patients with hypercholesterolemia, experts agree on the benefits of diet, physical exercise, and drug therapy in primary prevention and, particularly, secondary prevention.3–5 Nonetheless, despite the recommendations and new lipid-lowering agents, total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels exceed the recommended targets in both Spain and Europe.2,6–9
One of the factors limiting target achievement in hypercholesterolemic patients is nonadherence to treatment, which affects both medication use and lifestyle recommendations.10
To address this situation, various strategies have been used to improve adherence and thus lipid parameter control in these patients. In patients with risk factors such as hypertension, adherence has been improved through a combination of written material, telephone calls, and mailed information on the disease.11,12 In individuals with dyslipidemia, adherence has been improved through telephone reminders13,14 and cards containing treatment information.15 Studies on adherence in dyslipidemia indicate that improvements are required in both drug adherence and adherence to the other recommendations to achieve control targets.10
Given that combined strategies to improve adherence show better results than individual interventions,16,17 we planned a strategy including interventions with proven ability to improve adherence.13–15 Thus, the main aim of this study was to evaluate the efficacy of an intervention to improve the degree of control in hypercholesterolemic patients involving a strategy combining delivery of written information, text messages, and registration cards for the degree of adherence as complementary measures to standard clinical care. In addition, the study assessed the ability of the intervention to improve adherence (pharmacological, diet, and exercise).
METHODSThe present randomized, parallel-group, multicenter clinical trial is registered at ClinicalTrials.gov (NCT02314663). Participants were selected from clinics in 8 health care centers in 3 health districts of 3 Spanish autonomous communities: Castile-La Mancha (Albacete), Aragon (Zaragoza), and Galicia (Vigo). We included individuals aged ≥ 18 years previously diagnosed with defined hypercholesterolemia (TC ≥ 250mg/dL)18 who were receiving standard treatment (drug-based or not) and attending the participating centers. We excluded patients who were unable to undergo follow-up during the intervention (due to illiteracy or lack of a mobile telephone), had a physical disability impeding participation, or had a severe organic or psychiatric chronic disease precluding follow-up. All individuals signed an informed consent form after receiving a thorough explanation of the study. The study was approved by the Ethics Committee for Clinical Research in the Albacete Health Care Area and was carried out according to the ethics guidelines for clinical trials (Spanish Royal Decree 223/2004) and the Declaration of Helsinki.
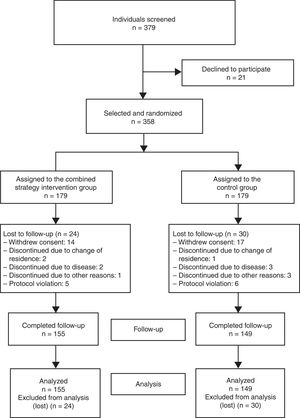
The sample size required for the analysis was calculated using a 2-sided test, and a difference of 20% in the proportion of participants achieving lipid control targets was considered clinically relevant: 55% in the control group19 and 75% in the intervention group. With 90% statistical power and 5% alpha error, a sample size of 155 individuals per group was estimated (310 total). After consideration of an expected patient loss of 15%, the final sample size per group was 179 individuals (total, 358). Of the 379 individuals asked to participate, 21 declined (acceptance rate, 94.5%) (Figure 1). The recruitment period lasted from March to December 2013.
For strategic reasons, 8 basic health care regions of the health districts of Albacete, Zaragoza, and Vigo were chosen for participant selection. To avoid carryover bias, participants in the control and intervention groups were from different areas. Thus, participant randomization was centrally performed according to health care region (Efron randomization) by a researcher who was not involved in the interviews or analysis. Participants were consecutively selected.
Participants in the intervention group received the following: a) written information on the disease and its treatment (provided at each visit) (Appendix 1 and Appendix 2 of the supplementary material); b) mobile telephone text messages with summaries of recommendations, reminders of dates of next appointments, and notifications of new appointments if any previous ones were missed (during between-visit periods) (Appendix 3 of the supplementary material); and c) self-completed registration cards on adherence to recommendations (during the entire follow-up) (Appendix 4 of the supplementary material). Both groups (intervention and control) received the standard recommendations of the European clinical practice guidelines for treatment of hypercholesterolemia and cardiovascular risk (CVR).3,20
Participants were followed up for a 2-year period, which finished in December 2015. Once patients were selected and provided consent, they were scheduled for the baseline visit. At this time, complete information was collected on medical history, physical examination, and blood tests. Patients received the standard treatment recommendations, both drug-based and nondrug-based. Together with the follow-up cards, participants from the intervention group were given written information on the disease and informed of the periodicity and content of the text messages that they would receive on their mobile telephones. The disease treatment reminders were sent every 15 days, whereas the attendance reminders for upcoming or missed appointments were sent according to the follow-up date. After the first visit, both groups attended 5 follow-up visits at the end of 2, 6, 12, 18, and 24 months. Variables were recorded at each visit but satisfaction information was only obtained during the final visit of the intervention group.
The main outcome variable was the proportion of individuals who achieved LDL-C target values according to European guidelines for dyslipidemias3 and CVR20 throughout a 24-month follow-up period. The plasma values indicated as targets were: a) LDL-C < 115mg/dL for patients without established cardiovascular disease or diabetes mellitus and with low or moderate CVR (SCORE < 5%); b) LDL-C < 100mg/dL for patients without established cardiovascular disease or diabetes mellitus but with organic disease and with high CVR (SCORE ≥ 5% and < 10%); and c) LDL-C < 70mg/dL for patients with diabetes mellitus or established cardiovascular disease and very high CVR (SCORE ≥ 10%).3,20 The lipid profile (TC, LDL-C, high-density lipoprotein cholesterol, and triglycerides) and its changes were also measured at each visit in all participants, who were informed of the results. Analytical determinations were performed using venous blood after a 12-hour fast. TC was determined using the CHODPAP method and LDL-C using the Friedewald method. Other variables examined were sociodemographic characteristics (age, sex, marital status, education level, and social class21), use of lipid-lowering agents (type, dosage), adherence to lipid-lowering therapy (Morisky-Green test22: adherence was considered good if patients responded correctly to the 4 dichotomous questions of the questionnaire regarding forgetting to take a dose and adherence to recommendations), adverse events, adherence to recommendations on lifestyle (adapted Morisky-Green), physical activity, and dietary habits, development of cardiovascular events (ischemic heart disease, atherothrombotic cerebrovascular disease, and peripheral arterial disease), anthropometric data, smoking, systolic and diastolic blood pressure, CVR (SCORE for low-risk countries and REGICOR),23 health problems (International Classification of Primary Care, Second Edition, World Organization of Family Physicians), use of other drugs, and degree of satisfaction with the combined strategy, assessed using a satisfaction questionnaire (Likert scale with 5 possible answers scored from 1 [very unsatisfied] to 5 [very satisfied]). The causes of study completion were a complete observation period (2 years), protocol violation, intercurrent disease precluding intervention continuance, and patient abandonment or consent withdrawal. No changes were made to the protocol during the study.
For statistical analysis, the variables of interest and stratification and potential confounding variables were compared between the 2 groups at study initiation. The homogeneity of the baseline values of the variables in the groups was evaluated (Student t test, chi-square). Subsequently, the individuals of both groups were classified according to LDL-C reduction or control, and a crude analysis evaluated the following parameters and their corresponding 95% confidence intervals (95%CIs): absolute benefit increase, relative benefit increase, and number needed to treat. In addition, the incidence of the outcome variable (proportion of individuals with adequate LDL-C control) was described and compared between the 2 groups in each follow-up period (comparison of proportions: chi-square). Changes in the lipid profile of each group throughout the study were compared using a Student t test or its nonparametric alternative (Mann-Whitney U test). Changes in the parameters in each group were analyzed using a repeated measures t test. The possible presence of other confounding factors and the interaction of other variables in the relationship between the proposed intervention and the outcome variables were evaluated using logistic regression models (dependent variable: control of lipid parameters). Continuous variables (LDL-C reduction) were evaluated using multiple linear regression. Efficacy analysis was performed via an intention-to-treat analysis that included all participants evaluated at 1 and 2 years. Analyses were performed using SPSS software (version 20.0).
RESULTSOf the 358 individuals who started the study, 155 and 149 completed follow-up in the intervention and control groups, respectively. There were no differences in the percentage of participants who completed the study between the groups (86.6% vs 83.2%; P = .376). The distribution of patients lost to follow-up is shown in Figure 1.
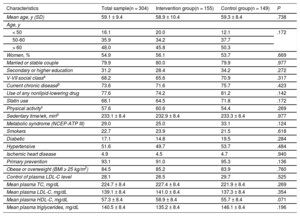
The patients’ baseline characteristics in both groups are shown in Table 1. The groups were homogeneous at baseline.
Baseline Characteristics of Participants Who Completed the Study and the Homogeneity in the 2 Groups
| Characteristics | Total sample(n = 304) | Intervention group(n = 155) | Control group(n = 149) | P |
|---|---|---|---|---|
| Mean age, y (SD) | 59.1 ± 9.4 | 58.9 ± 10.4 | 59.3 ± 8.4 | .738 |
| Age, y | ||||
| < 50 | 16.1 | 20.0 | 12.1 | .172 |
| 50-60 | 35.9 | 34.2 | 37.7 | |
| > 60 | 48.0 | 45.8 | 50.3 | |
| Women, % | 54.9 | 56.1 | 53.7 | .669 |
| Married or stable couple | 79.9 | 80.0 | 79.9 | .977 |
| Secondary or higher education | 31.2 | 28.4 | 34.2 | .272 |
| V-VII social classa | 68.2 | 65.6 | 70.9 | .317 |
| Current chronic diseaseb | 73.6 | 71.6 | 75.7 | .423 |
| Use of any nonlipid-lowering drug | 77.6 | 74.2 | 81.2 | .142 |
| Statin use | 68.1 | 64.5 | 71.8 | .172 |
| Physical activityc | 57.6 | 60.6 | 54.4 | .269 |
| Sedentary time/wk, mind | 233.1 ± 8.4 | 232.9 ± 8.4 | 233.3 ± 8.4 | .977 |
| Metabolic syndrome (NCEP-ATP III) | 29.0 | 25.0 | 33.1 | .124 |
| Smokers | 22.7 | 23.9 | 21.5 | .618 |
| Diabetic | 17.1 | 14.8 | 19.5 | .284 |
| Hypertensive | 51.6 | 49.7 | 53.7 | .484 |
| Ischemic heart disease | 4.9 | 4.5 | 4.7 | .940 |
| Primary prevention | 93.1 | 91.0 | 95.3 | .136 |
| Obese or overweight (BMI ≥ 25 kg/m2) | 84.5 | 85.2 | 83.9 | .760 |
| Control of plasma LDL-C level | 28.1 | 26.5 | 29.7 | .525 |
| Mean plasma TC, mg/dL | 224.7 ± 8.4 | 227.4 ± 8.4 | 221.9 ± 8.4 | .269 |
| Mean plasma LDL-C, mg/dL | 139.1 ± 8.4 | 141.0 ± 8.4 | 137.3 ± 8.4 | .354 |
| Mean plasma HDL-C, mg/dL | 57.3 ± 8.4 | 58.9 ± 8.4 | 55.7 ± 8.4 | .071 |
| Mean plasma triglycerides, mg/dL | 140.5 ± 8.4 | 135.2 ± 8.4 | 146.1 ± 8.4 | .196 |
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NCEP-ATP III, National Cholesterol Education Program-Adult Treatment Panel III; SD, standard deviation; TC, total cholesterol.
Unless otherwise indicated, the data represent percentage or mean ± standard deviation.
Semiskilled or unskilled manual workers (from industry, trade, services, and primary sectors) and homemakers.
Current chronic disease: presence of chronic disease included in the second edition of the International Classification of Primary Care diagnosed before study initiation and still present at the time of analysis.
Differences in lipid parameters at 1 and 2 years within groups and between the intervention and control groups are shown in Table 2. Comparison of the changes in the lipid parameters between the 2 groups during follow-up revealed a greater mean reduction in LDL-C and TC in the intervention group at both 1 year and 2 years. The mean LDL-C values (mg/dL) were 124.9 ± 37.0 and 119.5 ± 36.5 in the control and intervention groups at 1 year and 122.7 ± 35.3 and 117.1 ± 33.4 at 2 years, respectively. There were no significant differences during follow-up between the 2 groups in terms of changes in anthropometric parameters, blood pressure, and CVR (Table 2).
Changes in Lipid Profile, Weight, and Systolic and Diastolic Blood Pressure and Differences in the Reduction in These Parameters at 1 and 2 Years Between the Intervention and Control Groups
| Variables | 1 y | 2 y | ||||
|---|---|---|---|---|---|---|
| Change since baseline | Difference in reduction | Change since baseline | Difference in reduction | |||
| Mean (95%CI) | Mean (95%CI) | P | Mean (95%CI) | Mean (95%CI) | P | |
| LDL-C, mg/dL | ||||||
| Intervention | –21.4 (–26.8 to –16.0) | 9.3 (1.5 to 17.1) | .019* | –23.8 (–30.1 to –17.5) | 9.2 (0.7 to 17.7) | .034* |
| Control | –12.1 (17.8 to –6.4) | –14.6 (–20.4 to –8.9) | ||||
| TC, mg/dL | ||||||
| Intervention | –22.1 (–28.0 to –16.1) | 9.1 (0.3 to 17.9) | .042* | –25.4 (–32.4 to –18.4) | 9.7 (0.4 to 19.1) | .041* |
| Control | –12.9 (–19.5 to –6.4) | –16.3 (–22.5 to –10.0) | ||||
| HDL-C, mg/dL | ||||||
| Intervention | 0.8 (–0.9 to 2.6) | 0.7 (–2.2 to 3.4) | NS | 0.1 (–1.6 to 1.9) | 0.1 (–2.6 to 2.7) | NS |
| Control | 0.2 (–2.0 to 2.4) | –0.1 (–2.0 to 1.9) | ||||
| Triglycerides, mg/dL | ||||||
| Intervention | –3.5 (–13.7 to 6.6) | –7.9 (–23.1 to 8.2) | NS | –4.2 (–15.1 to 6.8) | –3.4 (–19.1 to 12.4) | NS |
| Control | –11.7 (–23.8 to 0.4) | –7.5 (–19.0 to 3.9) | ||||
| Weight, kg | ||||||
| Intervention | –0.41 (–1.09 to 0.29) | 0.51 (–0.62 to 1.64) | NS | –0.54 (–1.26 to 0.18) | 0.56 (–1.05 to 2.16) | NS |
| Control | 0.12 (–0.80 to 1.01) | 0.02 (–1.45 to 1.49) | ||||
| SBP, mmHg | ||||||
| Intervention | –1.90 (–4.18 to 0.38) | 0.84 (–2.50 to 4.19) | NS | –2.97 (–5.39 to –0.56) | 0.83 (–2.67 to 4.33) | NS |
| Control | –1.05 (–3.52 to 1.42) | –2.14 (–4.69 to 0.41) | ||||
| DBP, mmHg | ||||||
| Intervention | –0.88 (–2.37 to 0.60) | 0.23 (–1.79 to 2.25) | NS | –1.68 (–3.24 to –0.13) | 1.64 (–0.55 to 3.84) | NS |
| Control | –0.65 (–2.04 to 0.73) | –0.04 (–1.60 to 1.52) | ||||
95%CI, 95% confidence interval; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NS, not significant; SBP, systolic blood pressure; TC, total cholesterol.
Mean LDL-C reductions vs baseline were 12.8% and 5.2% (95%CI, 2.1%-13.3%; P = .007) in the intervention and control groups at 1 year and 13.1% and 6.4% (95%CI, 0.4%-13.0%; P = .038) at 2 years.
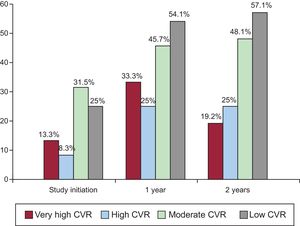
The proportion of individuals who achieved an LDL-C reduction was significantly higher in the intervention group at 1 year (relative risk [RR], 1.30; 95%CI, 1.11-1.51) and 2 years (RR, 1.17; 95%CI, 1.02-1.36). The proportion of individuals with adequate LDL-C control was significantly higher in the intervention group than in the control group at 1 year (RR, 1.46; 95%CI, 1.08-1.97). This proportion was nonsignificantly higher at 2 years (43.4% vs 34.7%; P = .119). At 1 year, the absolute benefit increase due to the achievement of LDL-C control targets was 14% (95%CI, 3%-24%), the relative benefit increase was 32% (95%CI, 8%-49%), and the number needed to treat was 7 (95%CI, 4-32). Post hoc analysis specifically considering the subgroup of patients with low or moderate CVR (SCORE < 5%) showed that the proportion of individuals with adequate LDL-C control was significantly higher in the intervention group at both 1 year (RR, 1.41; 95%CI, 1.02-1.94) and 2 years (RR, 1.39; 95%CI, 1.02-1.90). This outcome was not seen in individuals with high or very high CVR. The percentage of patients in the intervention group achieving the treatment target in each CVR subgroup at baseline and at 1 and 2 years are shown in Figure 2 (Appendix 5 of the supplementary material, patients with targets achieved according to CVR group).
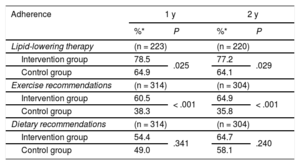
Adherence to statin therapy was significantly higher in the intervention group at both 1 year (RR, 1.21; 95%CI, 1.02-1.43) and 2 years (RR, 1.20; 95%CI, 1.02-1.43). There was also greater adherence to exercise recommendations in this group at 1 year (RR, 1.58; 95%CI, 1.24-2.01) and 2 years (RR, 1.81, 95%CI, 1.42-2.32). There were no significant within-group differences in adherence to dietary recommendations, although adherence was somewhat higher in the intervention group. The changes in the percentage of adherence during follow-up are shown in Table 3.
Change in Treatment Adherence in Participants Who Remained in the Study at Each of the Scheduled Visits Via the Morisky-Green Test
| Adherence | 1 y | 2 y | ||
|---|---|---|---|---|
| %* | P | %* | P | |
| Lipid-lowering therapy | (n = 223) | (n = 220) | ||
| Intervention group | 78.5 | .025 | 77.2 | .029 |
| Control group | 64.9 | 64.1 | ||
| Exercise recommendations | (n = 314) | (n = 304) | ||
| Intervention group | 60.5 | < .001 | 64.9 | < .001 |
| Control group | 38.3 | 35.8 | ||
| Dietary recommendations | (n = 314) | (n = 304) | ||
| Intervention group | 54.4 | .341 | 64.7 | .240 |
| Control group | 49.0 | 58.1 | ||
Table 4 shows the distribution of patients in terms of drug use at baseline and the changes in treatment during follow-up, with no differences between the 2 groups. There were no differences in the adverse effects of statins (7 in the intervention group and 10 in the control group). No patients had intervention-related adverse effects.
Distribution of Patients According to the Lipid-lowering Drug Taken at Baseline and Therapy Changes Made During Follow-up
| Use of lipid-lowering agents at baseline | ||||
|---|---|---|---|---|
| Total (n = 243) | Intervention (n = 114) | Control (n = 129) | P | |
| Simvastatin | 153 (63.0) | 74 (64.9) | 79 (61.2) | .554 |
| Atorvastatin | 67 (27.6) | 32 (28.1) | 35 (27.1) | .870 |
| Other | 23 (9.5) | 8 (7.0) | 15 (11.6) | .220 |
| Changes in lipid-lowering therapy during follow-up | ||||
|---|---|---|---|---|
| Total (n = 243) | Intervention (n = 114) | Control (n = 129) | P | |
| Change in lipid-lowering agent dose or type | 31 (12.7) | 15 (13.3) | 16 (12.2) | .804 |
| Change in dose | 17 (6.9) | 7 (6.1) | 10 (7.6) | .633 |
| Change in drug | 14 (5.7) | 8 (7.0) | 6 (4.6) | .412 |
| Total (n = 358) | Intervention (n = 179) | Control (n = 179) | P | |
|---|---|---|---|---|
| Change in drug dose or type or addition of new drug | 38 (10.6) | 17 (9.5) | 21 (11.7) | .493 |
| Addition of new drug | 7 (2.0) | 2 (1.1) | 5 (2.8) | .252 |
Unless otherwise indicated, the data represent No. (%).
At 1 and 2 years, 74.0% (95%CI, 66.8%-81.3%) and 90.8% (95%CI, 85.9%-95.7%) of intervention group participants reported being satisfied or very satisfied with the intervention.
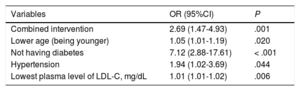
The factors related on multivariate analysis to a greater reduction in LDL-C values (multiple linear regression) at 1 year and 2 years are shown in the Table of the supplementary material (Variables y reducción de cLDL [Variables and Reduction in LDL-C]). The variables associated in the logistic regression with adequate control of LDL-C levels at 1 year are shown in Table 5.
Logistic Regression Model for Variables Related to Adequate Control of Plasma Low-density Lipoprotein Cholesterol at 1 Year
| Variables | OR (95%CI) | P |
|---|---|---|
| Combined intervention | 2.69 (1.47-4.93) | .001 |
| Lower age (being younger) | 1.05 (1.01-1.19) | .020 |
| Not having diabetes | 7.12 (2.88-17.61) | < .001 |
| Hypertension | 1.94 (1.02-3.69) | .044 |
| Lowest plasma level of LDL-C, mg/dL | 1.01 (1.01-1.02) | .006 |
95%CI, 95% confidence interval; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio.
Although the fundamental objective of dyslipidemia treatment is a reduction in LDL-C levels3–5 and achievement of the treatment targets recommended in clinical practice guidelines,4,5 there is still much room for improvement in lipid parameter control.6,24 The results of the present study show that, compared with the behavior recommended in the clinical practice guidelines, the combination of the combined intervention (delivery of written material, text messages, and self-completed registration cards) and the standard treatment was associated with a greater reduction in LDL-C and improved adherence to lipid-lowering therapy and exercise recommendations at 2 years; in contrast, the combined strategy was only related to improved LDL-C at 1 year of follow-up because this improvement was not significant at 2 years. These results could be due both to the difficulty of target achievement in patients with higher CVR—as indicated in Spanish and European studies25,26—and the levels considered targets for the higher-risk groups, given that, when analysis was restricted to the subgroup of patients with low or moderate CVR (SCORE < 5% at 10 years), superior control was seen in the intervention group at 2 years. In addition, this result might have been influenced by the high percentage of participants with a CVR < 5%, as well as the absence of an effect of the intervention on adherence to dietary recommendations and its possible influence on lipid control.
Our results are in agreement with both those of previous studies showing improved adherence without better LDL-C control27 and those showing reduced LDL-C without better control.28 Other interventions with fewer participants and inferior follow-up showed adherence and control improvements,13–15 similar to the results obtained here at 1 year of follow-up. Studies have also shown better lipid profile control without improved adherence.29 In addition, our adherence results are in agreement with those obtained in previous trials that improved adherence to exercise recommendations but not to diet.29
In addition to the type of intervention, differences from previous studies include the sample size, duration, and LDL-C values considered to indicate adequate control. The present study reports the long-term efficacy results of a multicenter trial of an intervention composed of 3 different strategies to reduce LDL-C and improve adherence and degree of control according to current European guidelines. Previous studies applied other interventions, often a single approach,14 with a smaller sample size and frequently with shorter follow-up than 6 months.13,15 We also determined the absence of adverse effects and high satisfaction with the intervention, variables omitted from previous studies.13–15,29
Regarding the clinical relevance of our results, the mean reduction in LDL-C and TC was not particularly relevant, although it was higher than that of previous studies that evaluated other interventions29 and could reduce the risk of cardiovascular morbidity and mortality.3 Thus, the benefit is due to both LDL-C control and reduced serum levels.30 In fact, LDL-C is the only variable without a threshold below which it no longer exerts a benefit,31 which is why some guidelines do not propose a specific LDL-C target.4 In addition, although the percentage of patients with adequate LDL-C control was low, the guidelines recommend the identification of more economic alternatives and implementation of prevention programs that engage patients and provide them with written and verbal instructions.3,20 Consequently, this intervention—which includes activities to improve dyslipidemia understanding, remind patients of indications, and facilitate greater patient participation—could be adapted to these recommendations and help to improve cholesterol control. Finally, although the reduction was not particularly relevant, these results indicate the value of this intervention in patients with hypercholesterolemia, allowing the use of lower statin doses and minimizing their possible secondary effects.
LimitationsOne of the most important study limitations is that the nature of the intervention precluded the use of participant blinding techniques; nonetheless, the results were evaluated in a blinded manner. There might also have been a difference in the risk of losses-to- follow-up between the groups, although the difference was not significant. Statin modification during follow-up could represent a limitation; however, because there were no differences between the groups, it is unlikely that the improved control or reductions in the lipid profile were due to changes in statin type or dose. In addition, the method used to determine adherence could be a limitation; however, there is no gold standard system that is both simple and reliable.32 The NICE (2009) guidelines33 also state that self-reported adherence is a useful tool for clinical practice. Moreover, the target measure was the LDL-C level, which is a clearly objective method. Finally, as in most trials, participation in the study possibly altered the behavior of both the physicians and the participants and possibly explains the improvements seen in the control group (Hawthorne effect).
New, longer controlled multicenter studies are probably necessary to ensure the ability of this strategy to decrease cardiovascular events. Also required are studies with greater power that are specifically designed to determine intervention efficacy in different CVR groups, as suggested by the results from individuals with low or moderate CVR. In addition, new strategies are needed that increase both pharmacological adherence and adherence to exercise and dietary recommendations because cardiovascular disease is still the leading cause of death, possibly due to the increased prevalence of unhealthy lifestyles.34,35 Cost-effectiveness studies of these interventions are also required that compare the different strategies to improve control and adherence.
CONCLUSIONSOur results show the ability of this combined and long-term intervention to reduce plasma LDL-C concentrations and improve treatment adherence in patients with hypercholesterolemia. Low-density lipoprotein cholesterol control was also improved at 1 year. The LDL-C reduction exceeded 10% vs baseline at both 1 year and 2 years, which could be clinically relevant.
FUNDINGThis study has received funding from the Instituto de Salud Carlos III and the Health Research Project Subprogram of the European Regional Development Fund (PI12/01955), resolution December 20, 2012.
CONFLICTS OF INTERESTNone declared.
- –
Low-density lipoprotein cholesterol control in patients with hypercholesterolemia requires improvement, although there is broad consensus on its treatment.
- –
The importance of treatment adherence in LDL-C control is reflected in the progressive increase in the space devoted to hypercholesterolemia in the successive guideline updates; it is now even one of their most important sections.
- –
Improvements in lipid profile rely on drug therapy and lifestyle modifications, with poor adherence affecting both factors.
- –
The present multicenter clinical trial has demonstrated the ability of an intervention to achieve a clinically relevant long-term reduction in plasma LDL-C concentrations.
- –
The results show long-term improvements in both pharmacological adherence and adherence to physical exercise recommendations.
- –
Our findings reveal better control in hypercholesterolemic patients through an intervention comprising 3 simple factors influencing behavior in patients with CVR.
We appreciate the participation of patients and staff.