Keywords
INTRODUCTION
During the past few years several reports have demonstrated the ability of adult bone marrow derived stem-cells to differentiate into several types of mature specific tissue-cells including cardiac myocytes and endothelial cells.1-5 This recognition has supported a series of studies in patients6-11 injected with bone marrow cells either intracoronary or directly at the borders of acute infarcts, resulting in a sustained degree of myocardial repair and functional improvement. In addition, myocardial regeneration via stem-cells mobilization at the time of experimental myocardial infarction has been known to occur12,13 and may be a further strategy for evaluation in humans. The feasibility and effects of this strategy in patients with acute myocardial infarction is currently under evaluation. This study shows the findings observed in a group of 13 patients with anterior wall acute myocardial infarction who were revascularized with stents and treated subcutaneously with G-CSF, aiming to improve ventricular function.
PATIENTS AND METHOD
Patients
We have prospectively studied 13 patients with revascularized anterior myocardial infarction who accepted to take part in a feasibility study on the effects of massive stem-cell mobilization to obtain myocardial regeneration in the infarcted area. The study was approved by the institutional Ethics and Research Committees and all patients gave signed informed consent. Table 1 shows the main baseline data. All 13 patients were initially treated with intravenous fibrinolytics on arrival, in an attempt to obtain early reperfusion. However, 2 patients needed rescue cardiac catheterization to restore perfusion as well as stent revascularization of the left anterior descending artery. The remaining 11 patients had non-invasive signs of reperfusion and stabilization and were scheduled for cardiac catheterization and stent revascularization between days 2 and 5 post-acute myocardial infarction. So, all patients underwent an initial cardiac catheterization between days 0 and 5 which was considered as the baseline condition for left ventricular function studies. In addition, concurrent myocardial revascularization during this procedure would ensure viability of the anterior wall and would prevent further scar tissue formation. Time from onset of pain to recanalization, stent revascularization and peak creatin-kinase were always recorded. After initial cardiac catheterization patients who agreed to enter the study were enrolled. Patients were treated with G-CSF and were closely monitored by frequent telephone calls after discharge. A second cardiac catheterization was scheduled at 3-month follow-up, to evaluate the changes in left ventricular function. The electrocardiograms obtained before discharge and at 3-month follow-up were compared. The summatory Q-wave (…Q), reflecting the degree of necrosis at the anterior wall, was measured and expressed in mm.
Diagnostic and Therapeutic Cardiac Catheterizations
Two cardiac catheterizations were performed during the study. The first was performed within the days 0 to 5 after acute myocardial infarction, the second at 3-month follow-up. In the first study a diagnostic left heart catheterization was undertaken, including left ventriculography and coronary angiography. Mechanical treatment of infarct-related left anterior descending artery was always performed. We implanted a sirolimus-eluting stent (Cypher, Johnson&Johnson, Cordis, Miami, Florida) at the site of occlusion. Then, coronary flow reserve (CFR) studies at the left anterior descending artery were performed to evaluate conditions in the capillary bed immediately after revascularization. In 3 patients additional stents were implanted at remote sites in non-related arteries. As a result, complete revascularization was always achieved. All therapeutic procedures were performed using an 8 Fr guiding catheter with no side holes. In the hemodynamic laboratory, the patients received 2 mg/kg of intravenous unfractionated heparin, continuing after the procedure with low-molecular-weight heparin (Dalteparin) 10.000 IU anti-Xa/day, ticlopidine 500 mg/day, and aspirin 150 mg/day, for at least 4 weeks, followed with clopidogrel and aspirin for 1 year. Five patients received standard doses of abciximab (0.25 mg/kg bolus followed by a 12-hour infusion of 0.125 μg/kg/min) starting during stent revascularization. Protamine was administered at the end of the procedure, following immediate removal of the femoral sheath with closure of the puncture site with Angioseal 8 Fr. Effective revascularization of the artery was always achieved. A new diagnostic cardiac catheterization was performed at 3-month follow-up. In this study, left ventricular angiography, coronary angiogram and CFR analysis were performed under identical conditions to those used at the initial study.
Left Ventricular Function Studies
In both conditions of the study we performed at least 1 left ventricular angiogram at 30º right anterior oblique projection. Angiograms were made through a digital General Electric system at a speed of 25 images/second. During each ventriculogram attempts were made to obtain a sinus beat together with a post-extrasystolic beat for later analysis, in order to determine contractile reserve behaviours of both14, infarcted areas (anterior wall) and compensatory hyperkinesis (inferior wall). Post-extrasystolic beats were obtained by inducing premature beats with the catheter, after filming a well opacified cardiac cycle of a normal sinus beat. In 3 patients, a second ventriculogram was performed at the end of the procedure in order to complete the capture of both kinds of beat.
Measurements and calculations were made off line in our own core lab where end-diastolic and end-systolic silhouettes were drawn. Left ventricular volumes and ejection fraction were calculated and regional wall motion was analyzed. The Sheehan method15 was used to study the extent of wall damage. The superimposed silhouettes were divided in 100 radii of wall shortening, from end-diastole to end-systole. The abnormal contracting segment (ACS) was defined as the percentage of radii showing akinesia or dyskinesia. The areas of the anterior wall affected by the infarction included the ACS plus the number of hypokinetic radii. Compensatory hyperkinesis was also identified, mostly at the inferior wall, and was expressed as a percentage of radii exhibiting hyperkinesis. Thus, the functional behaviour of the infarcted myocardium after treatment was serially observed and possible recovery could be evaluated. Functional recovery after treatment was defined as the gain in EF and the reduction of the infarcted area (ACS and number of affected radii) from baseline to 3-month follow-up left ventricular angiograms. In addition, the behaviour of the compensatory hyperkinetic zones was also examined.
Measurements of Velocities and Coronary Flow Reserve
The FloMap® system (Cardiometrics; Mountain View; California) was used for all velocity and CFR measurements.16 After successful revascularization treatment of the left anterior descending artery, a 0.014" intracoronary Doppler guide wire was positioned distal to the stented lesion and flow velocity was recorded continuously. The averaged peak velocity was obtained at baseline condition and after an intracoronary bolus of 60 μg of adenosine. Coronary flow reserve was calculated as the ratio between maximal flow velocity during the peak effect of the adenosine injection and the basal flow velocity. At 3-month follow-up, the same Doppler study following the same methodology was repeated in all patients. CFR was also calculated. Functional recovery of the infarcted microvasculature bed was defined as the gain in CFR from acute phase to 3-month measurements.
G-CSF Mobilization and Laboratory Studies
Patients received G-CSF (Neupogen, Amgen, Thousand Oaks, CA) subcutaneously for 10 days at a dose of 5 μg/kg every 12 hours. Samples of peripheral blood were taken on days 0, 3, 5, and 10 for determination of total white blood cell count, percentage of circulating CD34+ cells, and immunophenotyping. Serial tests of cardiac enzymes were performed during the acute phase and during G-CSF treatment.
Quantification of G-CSF mobilized peripheral blood CD34+ cells was performed in compliance with ISHAGE guidelines.17 Three-color immunofluorescence cytometry was used to assess the expression of different molecules on mobilized peripheral blood CD34+ cells. Cells were stained with peridin chlorophyll protein (PerCp)-labelled monoclonal antibody (MnAb) against human CD34 (HPCA-2, Becton Dickinson, San Jose, CA), and the following fluorescein isothiocyanate (FITC) or phycoerythrin (PE)--labelled mouse anti-human MnAbs: Anti-CD33 (clone WM 53), Anti-CD117 (c-kit, clone YB5.B8), anti-CD41a (Clone HIP8), anti-CD71 (clone M-A712), anti-fusin (CXCR4, clone 12G5), anti-CD90 (Thy-1, clone SE10), anti-HLA-DR (clone G46-6) (all from Pharmingen, Becton-Dickinson, San Jose, CA), and anti-CD38 (clone T16) (from Immunotech, Marseille, France). Cells were harvested and analyzed in a dual-laser FACScalibur flow cytometer with Cell-Quest Software (Becton-Dickinson). Forward scatter versus side scatter signals were used to discriminate hematopoietic cell population from erythrocytes and debris. CD34+ population was gated in a FL3 versus side scatter dot plot. The percentage of positive cells was calculated using appropriate isotype controls. At least 2000 CD34+ events were collected for analysis.
Statistical Study
Data are expressed as mean ± standard deviation. A Student-Fisher paired t test was used to compare means from the same patients. Linear correlation between variables was analyzed with the Pearson test. A value of P<.05 was considered statistically significant.
RESULTS
Clinical and Laboratory Findings
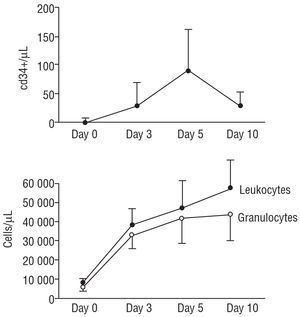
All patients survived the acute myocardial infarction. Four of them needed inotropic drugs during the acute phase. None of them developed complications secondary to any cardiac catheterization procedure performed during the hospital phase or at follow-up. No further increases in enzymes were detected during G-CSF treatment. Patients showed significant elevation of circulating leukocytes during G-CSF administration together with a significant increase in the total number of circulating CD34+ cells that reached its maximum on day fifth after the start of the treatment. Figure 1 shows the effects of G-CSF on peripheral white blood cells counts and total number of CD34+ circulating cells. Immunophenotypical analysis of circulating CD34+ cells on fifth day demonstrated that although many cells express typical hemopoietic lineage markers (CD33, CD117, CD41, CD71), many others have characteristics of very immature stem-cells (CD38-, HLA-DR-, CD90+), rendering a worthwhile number of total circulating CD34+ cells theoretically pluripotent. The percentages and the total numbers of the distinct subpopulations of CD34+ circulating cells are shown in Table 2. All patients were discharged symptom-free with a regimen of vasodilators and antithrombotics. Adverse effects of G-CSF were mild fever or muscular pain which were well tolerated by all patients. However a major adverse effect was recorded in 1 patient who suffered spontaneous spleen rupture and emergency splenectomy was performed 13 days after acute myocardial infarction while still under G-CSF administration. The factor was discontinued and the patient did well after surgery. Pathologic examination showed severe acute splenitis with subcapsular haemorrhage as the cause of rupture. After a mean clinical follow-up of 9±2 months, all 13 patients remain symptom free and continue vasodilatory and antiplatelet medications. No episodes of angina or heart failure have been recorded to date. The mean …Q-wave obtained from the electrocardiogram decreased significantly at follow-up study (Table 3), suggesting some degree of electrical recovery (Figure 2).
Figure 1. Serial determination of absolute number of CD34+ cells (upper panel) and leukocytes and granulocytes (lower panel) during treatment with G-CSF. A peak concentration of CD34+ is observed at day 5.
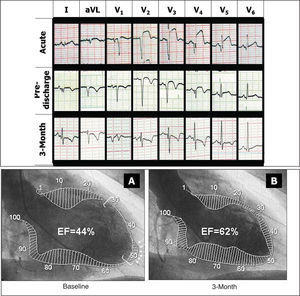
Figure 2. Serial electrocardiograms and ventriculograms obtained from a patient with acute myocardial infarction. A significant functional recovery and a reduction of necrotic Q-wave can be observed. Baseline (A) and 3-month follow-up (B) ventriculograms from a patient. Arrows show dyskinetic segment at the apex. Brackets delineate the abnormal contracting segments (ACS).
Hemodynamic and Angiographic Findings
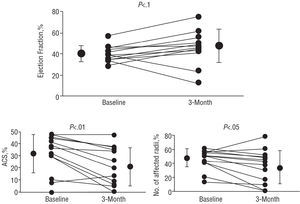
Table 3 also summarizes the main changes observed in global and regional left ventricular function, from acute phase to 3-month follow-up. As can be seen, there were no significant changes in volumes. However, a significant improvement in global and regional function was observed at follow-up (Figure 3). The % of ACS and of affected radii at the anterior wall in sinus beats became significantly reduced at follow-up, with no changes in compensatory zones at the inferior wall. This led to a mild, but not significant, increase in global ejection fraction. Similarly, post-extrasystolic beats also showed significant improvements in the contractile capacity of the anterior infarcted wall. CFR of the infarcted territory also improved significantly, from acute phase to follow-up, recovering normal responses to vasodilatory stimulus.
Figure 3. Individual changes observed in ejection fraction (EF), abnormal contracting segment (ACS), and number of affected radii at the anterior wall.
Determinants of Functional Recovery
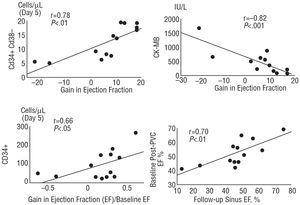
The absolute gain in ejection fraction oscillated between -22 and +18% (mean, 6.2±12.3) and correlated significantly (r=0.71; P<.006) with the gain in the % of recovered radii in the anterior wall. Thus, although global functional recovery varied among patients, it mostly depended on the changes observed in the infarcted anterior wall. Figure 2 shows an example of a significant functional recovery after stent revascularization followed by a 10-day course of G-CSF. However, 2 patients showed a clear worsening in global left ventricular function at 3-month follow-up. Thus, failure in functional recovery following our strategy was revealed in 15%. Factors influencing functional recovery were investigated (Figure 4). The gain in ejection fraction was inversely related to the baseline left-ventricular end-diastolic pressure (r=-0.56; P<.05) and to the peak mb creatin-kinase (r=-0.82; P<.002), both suggesting an adverse influence for recovery in those patients with greater infarct size (extent). On the contrary, the gain in ejection fraction directly correlated with the total number of circulating CD34+ CD38- cells/μL on the fifth day of G-CSF treatment (r=0.78; P<.003). In addition, total circulating CD34+ cells/μL correlated with % of gain in ejection fraction (r=0.66; P<.05). The baseline post-extrasystolic ejection fraction correlated significantly (r=0.70; P<.01) with the sinus beat ejection fraction at follow-up. No time parameters during the acute phase had significant influence on functional recovery after treatment.
Figure 4. Significant correlations obtained from our study.
The baseline CFR, obtained immediately after stent placement, inversely correlated with the baseline % of ACS (r=-0.75; P<.003). Regarding functional recovery of the capillary bed at the infarcted area, the absolute gain in CFR oscillated between -0.5 and +2.2 (mean, 0.98±0.62). This increase did not correlate with baseline left ventricular function parameters and was irrespective of the change in ventricular function. Changes in CFR did not correlate with the total number of circulating CD34+CD38- cells/μL on the fifth day of G-CSF treatment.
DISCUSSION
This study shows the feasibility and safety of stem-cells mobilization in patients with acute myocardial infarction and suggests that G-CSF treatment could influence ventricular function recovery. The concept of myocardial regeneration after acute myocardial infarction via stem-cell mobilization was first demonstrated by Orlic et al12 in an experimental murine model. In addition, it has been shown in non-human primates that cell mobilization by hemopoietic growth factors promote angiogenesis in the infarcted myocardium.13 However, no detectable myocardial repair was observed in this experimental model where reperfusion was not considered mandatory. The nature and number of mobilized cells might also differ among mammalian species. These findings in animal models have generated expectations on the possible clinical application and at present there is growing interest but little available information on the feasibility, safety and efficacy of stem-cell mobilization with G-CSF in patients with acute myocardial infarction. Strategies, timing, dosage and duration of treatment with G-CSF after an acute myocardial infarction are empiric and may vary according to the design of research currently under way. In a recent study of 10 patients, Kang et al18 studied the effects of the intracoronary infusion of mobilized stem-cells at the time of stent revascularization, which had been obtained previously by apheresis. At follow-up they observed a favourable change in left ventricular function, despite an adverse influence on the high restenosis rate at the treated culprit lesion. The authors suggest that this unexpectedly high restenosis rate might be caused by the differentiation of progenitors into smooth muscle cells within the stented segment. This adverse finding, if secondary to stem-cells mobilization, could limit the efficacy of this treatment and create new and unexpected concerns. However, no restenosis was observed in our patients.
In our feasibility study of patients with anterior myocardial infarction, early reperfusion and stent revascularization was always obtained as a pre-requisite for selection, in 2 of them as a rescue reperfusion procedure. Thus, stent scaffolding of the culprit coronary lesion was performed before G-CSF treatment. Since a 10-day course of 10 μg/day of G-CSF was started 5 days after acute myocardial infarction, and peak circulating concentrations of progenitor cells were observed at the fifth day of G-CSF treatment, circulating stem-cells might have fewer opportunities to differentiate into smooth muscle cells within the stented segment; mainly if peak concentrations do not coincide in time with the fresh stent-covering over a complicated coronary plaque. This time difference, together with the use of sirolimus-eluting stents in our patients may account for the differences seen in restenosis rates. However, angiographic follow-up was performed at 3 months. Thus, our patients remain under close evaluation for the possibility of late residual anterior wall myocardial ischemia.
Another point of interest is the ideal dosage and length of course for G-CSF. We selected a 10-day course starting 5 days after acute myocardial infarction, trying to separate peak concentrations of circulating mobilized stem-cells from acute conditions by a few days. This could, in theory favour myocardial homing and differentiation6. In addition, no mobilized cells were collected for further coronary infusion but instead, massive and sustained stem-cells mobilization was performed expecting self myocardial homing as a response to chemoattracting cytokines.3,5,19 The selected dosage of G-CSF is universally accepted for mobilization20-22 and has been widely used in our center in hemopoietic transplant donors. Serial laboratory determinations confirmed the persistent cell mobilization up to tenth day after the beginning of G-CSF, and therefore 15 days after acute myocardial infarction. Thus, a worthwhile amount of immature stem-cells (CD38-, HLA DR-, CD90+) circulate in peripheral blood 10 days after myocardial infarction creating an opportunity for homing at the infarcted myocardium. In addition, G-CSF could also influence resident cardiac stem-cells to divide and differentiate.
We had an unexpected complication in one patient who developed spontaneous spleen rupture at day 8 of G-CSF treatment due to acute splenitis and subcapsular hemorrhage. Mobilized leukocytes may be partially captured by the spleen23-25 but spontaneous rupture, although described elsewhere,26-28 is a very rare complication after G-CSF administration in hematologic patients or healthy donors. Our patient was successfully operated and experienced significant functional improvement at follow-up (gain in EF: 13%). However, the possibility of acute splenitis induced by massive cell mobilization should be addressed with caution, mainly considering that subcapsular hemorrhage may be favoured by the simultaneous antithrombotic regimen needed to avoid subacute stent occlusion.29
Finally, the study of the effects these strategies have on myocardial regeneration continues to raise difficulties for clinical investigation. Since early reperfusion and stent revascularization are needed to warrant adequate flow in the infarcted territory, it seems quite difficult to differentiate the changes in left ventricular function secondary to salvage of jeopardized myocardium at risk from improvements gained by successful myocardial regeneration. Mainly, if we consider that the degree of stem-cells mobilization may vary among patients and is not always efficient for a gain in EF. Worsening of global left ventricular function may also occur18 in a small percentage (15% in our experience). The reasons for this worsening are unknown and seem to involve multifactorial influence, including reperfusion injury or even cell microinfarctions.30 Further randomized studies are needed to clearly separate the effects of reperfusion from those provided by myocardial regeneration. In the meantime, our findings suggest that functional recovery of the anterior infarcted wall could be favoured when high peak concentration of immature circulating mobilized cells occurs on the fifth day of G-CSF treatment. Some electrical recovery, as determined by the Σ of Q-wave of the surface electrocardiogram, may be also observed, which could suggest some degree of electrogenesis at the anterior infarcted wall. Our serial angiographic study on left ventricular function shows significant improvements in global ventricular function parameters and regional wall motion, not only in normal sinus beats but also in post-extrasystolic beats. This would suggest a further recovery of post-extrasystolic potentiation at the infarcted anterior wall, which may be translated into preservation of contractile reserve.14,31 However, this functional benefit may be limited in patients presenting with greater infarct size.
CONCLUSION
In conclusion, stem-cells mobilization with G-CSF is a feasible and safe treatment for attempting myocardial regeneration in patients with revascularized AMI. However, the possibility of acute splenitis induced by massive cell mobilization should be taken into account in further study designs using this strategy.
Correspondence: Dr. J. Suárez de Lezo.
Servicio de Cardiología. Hospital Universitario Reina Sofía.
Avda. Menéndez Pidal, 1. 14004 Córdoba. España.
E-mail: grupo_corpal@arrakis.es