Keywords
INTRODUCTION
The 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) have, in addition to their hypolipidemic action, other effects which have clinical benefits. Among these pleiotropic effects,1 their antiinflammatory action2 became a focus of interest due to the physiopathological role that inflammation plays in coronary artery disease.3 Thus, growing interest exists in the use of these drugs to attenuate the inflammatory response, both in primary prevention4 and in stable coronary disease5,6 as well as within the framework of acute coronary syndrome (ACS)7 where, in addition to reducing inflammatory levels,8 the early administration of statins is associated with a more favorable clinical evolution.9 In fact, the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT) study10 shows how, in patients with ACS, intense and early therapy with statins, with a decrease in low-density lipoprotein (LDL) values below current objectives, is associated with greater cardiovascular protection at 24-month follow-up.
The prognostic value of systemic inflammatory markers has led to their widespread use when stratifying cardiovascular risk.11 In particular, C-reactive protein12 (CRP), a protein with great atherogenic potential, has been identified as the parameter with the greatest clinical application due to its predictive power in primary prevention, chronic ischemic heart disease,6 ACS without ST segment elevation12 and ACS with ST segment elevation.13 Interleukin 6 (IL-6) is the main stimulus for the synthesis of CRP in the liver and is a key cytokine in the inflammatory response.3 The values of IL-6 are significantly correlated with those of CRP14 and also have prognostic value in coronary artery disease.15
Recently, several studies5,6,8,16,17 have confirmed the antiinflammatory effect of statins based on the reduction in CRP values. However, these works analyze the role of the drug on this acute-phase reactant in primary prevention, in stable coronary heart disease and, to a lesser extent, in ACS without ST segment elevation. However, there are no data on ACS with ST segment elevation. The aim of the present study was to evaluate the effect of early administration of pravastatin on blood levels of CRP and IL-6 in the framework of acute myocardial infarction (AMI) with elevated ST segment, as well as its possible effect on the clinical evolution of these patients.
PATIENTS AND METHOD
Population and Study Design
Between January 2001 and September 2002, 71 consecutive patients with ACS with ST segment elevation were prospectively studied. Patients were included in the study if they had anginal thoracic pain of over 30 min duration and ST segment elevation ≥0.2 mV in at least 2 contiguous ECG leads. Immediately before the reperfusion treatment (percutaneous or thrombolytic, depending on the decision of the physician who received the patient), patients were single-blind randomized to receive 40 mg/day pravastatin (group treated, n=35) or not (control group, n=36). The study was not placebo-controlled. Patients were assigned to each group within the 10 h following the beginning of pain. Exclusion criteria were: patients under 18 years old, those who had had previous heart attack, previous hypolipidemic treatment, cardiogenic shock upon admission, neoplastic disease, known kidney failure, known intolerance to statins, known liver failure, a background of cardiomyopathy, and the presence of any infectious or inflammatory process upon admission, or which appeared during the study period. The size of the sample was calculated from the results of the first 25 patients (pilot study), with an α risk =.05 and β=.10 to detect a reduction in CRP of 30% after 7 days of treatment, and it was estimated that there would be 7% loss.
Upon discharge, all the patients received optimized treatment and a low-calorie low-fat diet. All patients were clinically reevaluated at 2 months following the event. A complete lipid study was then repeated and pravastatin treatment begun in the patients in the control group who presented LDL values more than 100 mg/dL.18
The study protocol was approved by the center's ethics committee center based on the information available regarding the optimal time to initiate treatment with statins after AMI with ST segment elevation.18
Laboratory Analysis
Venous blood was extracted, and after centrifugation serum samples were frozen at -70°C for later analysis. Lipid values were studied on admission, at 7 days and at 2 months after the acute episode, in an automated chemistry system (Advia 1650, Bayer, Tarrytown, NY). The LDL value was calculated with Friedewald's formula.
The concentrations of IL-6 were measured with ELISA test kits (Quantikine Human IL-6, R&D Systems, Minneapolis, MN) following the manufacturer's instructions (range: 3.13-300 pg/mL). C-reactive protein was analyzed through a high sensitivity ELISA test (Generic Assays, Dahlewitz, Germany), that has a detection range of 0.56 mg/L to 200 mg/L. In both cases the analysis was carried out in serum collected in the acute phase (<10 h), and at 2 days and 7 days after the ischemic episode. In addition, at 2 months the CRP values were determined through turbidimetry (range: 5-200 mg/L); the patients were classified according to whether their concentrations were greater or less than 6.6 mg/L, in line with risk stratification according to the Cholesterol And Recurrent Events (CARE) study19. The blood samples were coded, which meant that the doctor who analyzed them did not know whether the patient had received pravastatin or not.
Clinical Events
The development of early clinical events was evaluated throughout hospitalization and during the first 2 months of follow-up. Combined angina, reinfarction or death was considered as the primary clinical event. Angina was defined as typical precordial pain with electrocardiographic changes and AMI as clinical angina and a double or more increase of creatine kinase with concomitant elevation of MB isoenzyme or troponin I. Death was defined as mortality due to both vascular and non-vascular causes.
Clinical events were evaluated by physicians unaware of the study design or the randomization process on admission, such that, insofar as possible, event allocation was done while blind to treatment received.
Statistical Analysis
Data were analyzed using the SPSS statistical package (version 10.05; SPSS Inc., Chicago, IL). Continuous variables were expressed as mean and standard deviation (SD), and as the median and semiquartile range when they were not adjusted to normal distribution; the qualitative variables were expressed as percentages. Comparison of means was carried out with Student's t tests or nonparametric Mann Whitney U-test for data without a normal distribution. The paired samples study was done with Student's t test for paired samples or with Wilcoxon's test for ranges with variable signs that did not have a normal distribution. The General Linear Model was used for repeated samples (MANOVA) in the study of markers when they related to the effect of the drug at different stages during the study. Spearman's rho was used to study the correlations. The percentages were compared with chi-squared and the odds ratio (OR) was expressed at a confidence interval (CI) of 95% for a two-tailed test. A P-value <.05 was considered significant.
RESULTS
Demographic Characteristics
The characteristics of the 71 patients included in the study are shown in Table 1. Fifty-eight patients treated with percutaneous coronary intervention were transferred to the referral hospital (Hospital Universitario Virgen de la Arrixaca) and in all cases TIMI III flow was obtained via angiography. Alteplase was administered as the thrombolytic. The mean time to randomization was 289.8±122.9 min, and there were no differences between the control group and the treated group (282.9±115.4 min vs 296.7±138.0 min; P=.61).
Lipids, C-Reactive Protein and Interleukin 6 Values
On admission, no significant differences were observed in lipid concentrations between the 2 groups. After 2 months of treatment, the total cholesterol and LDL values were significantly lower in the treated group; in fact, these differences were already significant by day 7 of the study. There were no differences between the 2 groups in high-density lipoprotein (HDL) and triglyceride values during the study period (Table 2).
Regarding the evolution of the lipid profile, it was found that in the control group the total cholesterol and LDL values increased significantly in the first 7 days; from this moment onward all lipid parameters increased except for triglycerides. In contrast, there were no significant changes in lipid concentrations during the first 7 days in the treated group, but between day 7 and 2 months a significant elevation was found in total cholesterol and HDL without changes in LDL and triglyceride values.
C-reactive protein values are shown in Table 3, panel A. These were similar in the 2 groups on admission.
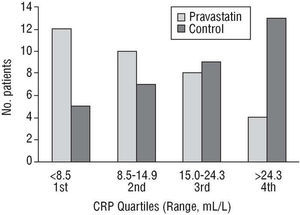
At 48 h, the increase in CRP values was smaller in the group treated with pravastatin but they did not reach statistical significance (P=.12). After 7 days of treatment, the CRP values were significantly lower (P=.002) in the group that received the drug. Median and mean CRP values were 48% and 59% lower compared to the untreated group, respectively. Thus, only 35.2% of the control group patients were below the median (14.9 mg/L) versus 64.5% of the patients in the treated group (Figure 1).
Fig. 1. Day 7: distribution of C-reactive protein (CRP) quartiles in both groups.
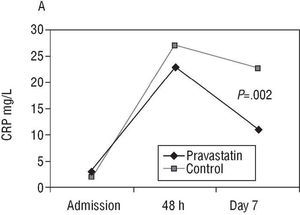
Considering the 71 patients as a whole, CRP increased significantly (P<.001) from admission until 48 h, subsequently presenting a significant decrease between that time and day 7 (P<.001) (Figure 2A). This decrease was more pronounced in terms of the mean and median (48.4% and 51.9%, respectively) in the treated group than in the control group (32.5% and 15.9%). At 2 months, 64 patients were still alive (32 in each group); 50% of the control group and 25% of the treated group presented a CRP value greater than 6.6 mg/L (P=.039).
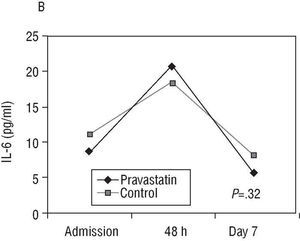
Fig. 2. Evolution of C-reactive protein (CRP) and interleukin 6 (IL-6) (median) in both groups.
The IL-6 values were not significantly different between the groups after 7 days of treatment (Table 3, panel B), nor was the mean decrease of IL-6 from 48 h to day 7 (Figure 2B) (73.2% in the control group and 68.9% in the treated group).
Correlation analysis in the treated group showed that the changes in CRP concentrations were independent of the changes in total cholesterol (P=.61), LDL cholesterol (P=.51), HDL cholesterol (P=.66), and triglycerides (P=.95). Nor was there any correlation between the changes in CRP and lipid parameters when the calculation was carried out in the patient population as a whole or in the control group.
In addition, when analyzing whether the variations in CRP concentrations could be accounted for by differences in IL-6 values (Table 4), we found that CRP and IL-6 values in the control group were significantly and increasingly tightly correlated as AMI evolved. However, in the treated group the values correlated upon admission (before receiving the drug) but this correlation stopped being significant in the first 48 h. Although at day 7 both parameters correlated, this dependency only reached a weak statistical value.
Clinical Events
Before discharge, 10 (14%) patients presented a cardiac event: 7 occurred in the control group and 3 in the pravastatin group (P=.18). These events included: 2 deaths in each group (3 from cardiogenic shock and multiorgan failure and 1 from cardiac rupture), 1 reinfarction in the control group and 5 postinfarction anginas (4 in the control group and 1 in the pravastatin group). In the following 2 months, 7 new events occurred in the control group (1 reinfarction, 4 anginas and 2 deaths, 1 sudden and the other due to cardiogenic shock) and only 2 in the pravastatin group (1 angina and 1 sudden death). No ictus occurred during follow-up. Globally, 16.9% of the control group versus 5.6% of the treated group experienced an event after 2 months follow-up (OR=0.258; 95% CI, 0.074-0.91; P=.027).
The mean concentrations of CRP were higher in the patients who developed events during the 2 months of the study, both at admission (8.2±3.5 vs 3.5±4.21 mg/L; P=.006) and at 48 h (39.1±29.7 vs 25.7±12.7 mg/L; P=.046). By day 7, the value was also higher in the patients who survived events, but without presenting statistical significance (23.9±12.6 vs 15.8±10.7 mg/L; P=.14).
DISCUSSION
The present study analyzes the effect of pravastatin on 2 inflammatory markers, CRP and IL-6. It was carried out within a clinical context which until now has been poorly studied, that is, AMI with elevated ST segment. Our data demonstrate that the early administration of a daily single dose of 40 mg of pravastatin is associated with significantly lower CRP values after 7 days of treatment and, accordingly, with a smaller increase in CRP during the acute phase of the myocardial infarction. Furthermore, after 2 months of treatment, CRP concentrations remain lower (<6.6 mg/L) in a greater percentage of cases compared to controls. This value, associated with an increase in risk in the chronic phase of AMI according to the CARE study,19 is reliably detected through a routine method such as turbidimetry. In addition, the early administration of the drug provides a better lipid profile after the first 7 days and at 2 months.
The results of the study support the use of the drug in ACS with ST segment elevation from the moment of diagnosis due to its capacity to attenuate the inflammatory response. Thus, taking into account the known prognostic value of CRP, according to our results, early administration of pravastatin would be justified in order to facilitate the decrease in CRP to chronic levels after AMI and help diminish the number of clinical events.
Unlike other works,9,10 in our study the administration of pravastatin was always carried out in the 10 h following the onset of the pain. This means that the antiinflammatory effect, and probably the rest of the pleiotropic effects, initiated its action immediately after closing the artery, having an effect on CRP after only 7 days of treatment. Curiously, the treatment with pravastatin did not modify the values of its regulator in the liver, IL-6, at 48 h or at day 7 of treatment.
Effect of Pravastatin on C-Reactive Protein. Previous Studies
The prognostic value of the CRP and its modification with statins has been evaluated in several studies, both in primary4,6,17,20,21 and in secondary prevention. In this regard, the CARE study5 showed a reduction in median CRP of 17.4%. Patients in the highest quintile (CRP≥6.6 mg/L) had a more than 80% probability of developing a coronary event during follow-up.19 There was also a significant reduction in CRP in The Pravastatin Inflammation/CRP Evaluation (PRINCE) study6 after 12 weeks of treat ment with pravastatin, in other studies with atorvastatin after 4 weeks22 and even after only 48 h.23 Retrospective observational studies present similar data.24 In ACS without ST segment elevation, the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering trial (MIRACL) study presented a higher mean reduction in CRP after 16 weeks in the treated group (P<.0001).8 In another study,16 also in patients with ACS without ST segment elevation, atorvastatin was associated with a decrease in CRP of 5% after 5 days of treatment and an increase of 188% in the placebo group.
Finally, there are no data concerning the effect of statins in the context of ACS with ST segment elevation. In our series, the median CRP was 48% lower in the treated group. We also found a decrease in clinical events, although this was limited by the small sample size. Postinfarction angina should be highlighted (2 in the treated group and 8 in the control group), as it is probable that the improvement in endothelial function,25 a known effect of pravastatin and probably also promoted by the decrease in CRP, represent the basis for the distribution of such event.
Our data, as well as those found in the medical literature, do not show a correlation between changes in CRP and LDL values, which means that the lowest value of oxidized LDL does not seem to be the cause of the low release of IL-6 by the macrophages and, thus, of CRP in the liver.3
Effect of Pravastatin on Interleukin 6 and Correlation With C-Reactive Protein
The concentrations of CRP correlate with IL-6 values.14 However, in our series, this correlation was only observed in the group that did not receive pravastatin. In contrast, in the treated group, the correlation between the 2 markers was weak at day 7 and nonexistent at 48 h. This could be explained by the drug not significantly modifying the IL-6 values, whereas it did modify those of CRP, which is in agreement with other studies.8,20,24
According to these results, the decrease in CRP levels can not be attributed to a decrease in IL-6 synthesis in peripheral tissues, since there levels are not modified after treatment. However, it is possible that the decrease in CRP without changes in IL-6 can be explained by the inhibitory effect of pravastatin on the synthesis of isoprenoids in the liver, reducing the activation of G-protein Rac-1 that mediates CRP transcription after stimulating IL-6 in the liver.7,26,27
Finally, it is possible that the normal doses of pravastatin in addition to its hydrophilic properties are two reasons why no cellular blockage of IL-6 synthesis in non-liver tissues takes place. However, other studies with high doses of atorvastatin (80 mg), a lipophilic statin, do not show a capacity to decrease IL-68 values.
Limitations
Given the small sample size, caution should be exercised when interpreting the clinical events. In this regard, limitations arose when randomizing; of note, the control group had more women and worse coronary anatomy, although without statistical difference. Nevertheless, neither the CRP and IL-6 values nor the total events were significantly different according to sex. In addition, the number of affected vessels was not associated with different IL-6 values, although on admission there were greater concentrations of CRP. Neither were there significant differences in the number of patients with disease in more than one vessel who developed events during the study.
On the other hand, given its short half-life and circadian variations, the IL-6 values should be interpreted with caution, as mentioned in previous studies.8 Nevertheless, in order to overcome these limitations, blood was extracted in a well-lit place,28 during the day and night, and the hour of extraction was adjusted to specific times during the evolution of the heart attack. Finally, we should take into account that correlational changes in LDL and CRP could be blocked by the behavior that LDL has in ACS as an acute-phase reactant.
CONCLUSIONS
The administration of 40 mg/day of pravastatin from the time of diagnosis of ACS with ST segment elevation is associated with lower CRP plasma concentrations after 7 days of treatment, and thus a smaller increase in its values when compared to admission. This effect is independent of changes in lipid values. This dosage of pravastatin does not produce substantial changes in IL-6 plasma concentrations, which support the inhibitory effect of the drug in the liver. Our results demonstrate better clinical evolution in the treated patients, although this should be corroborated by new and larger patient population studies.
ACKNOWLEDGEMENTS
The authors thank the registered nursing staff M. Jesús Cáscales and M. José G. Villalba for their work, and to laboratory technician M. Huertas González for assistance in processing samples. We also thank Dr. Andrés Carrillo for his useful advice regarding the statistical treatment of the data.
This study was funded by the Association for Heart Disease Prevention (PRECOR). Murcia GIF:G-73022121.
Correspondence: Dr. Manuel Gonzálvez Ortega.
Unidad de Cardiología. Hospital Universitario Morales Meseguer.
Avda. Marqués de los Vélez, s/n. 30008 Murcia. España.
E-mail: manu.mur@terra.es