Keywords
INTRODUCTION
Compared with bare metal stents (BMS), drug-eluting stents (DES) reduce the incidence of restenosis and the subsequent need for new revascularization procedures.1,2 On the other hand, DESs increase the risk of stent thrombosis (ST) compared to BMS, especially after the first year (very late ST).3-8
However, the fact that clinical trials are performed in highly selected patients may mean that their meta-analysis underestimates the incidence of ST with DESs. In the real world, where patients with complex medical conditions (myocardial infarction, renal failure, ventricular dysfunction) or complex lesions (bifurcated lesions, restenosis, surgical grafts, small vessels) are common, the incidence of ST with DESs should logically be greater.9 This excess risk of ST could offset the clinical benefits of DESs implied by a reduction in restenosis.
In this context, we sought to compare the risk of ST with DES and BMS and to investigate its possible clinical consequences in routine clinical practice. We also specifically aimed to assess the effect of very late ST on the risk-benefit ratio after implantation of a DES.
METHODS
Study Design
We performed a retrospective analysis of clinical outcomes in 2 cohorts composed of all consecutive patients treated with at least one DES or one BMS in the study center's cardiac catheterization lab during 2003-2004. Patients who received both types of devices were excluded from the study. During that time, paclitaxel-eluting stents (PES) were the only DES available in the study center, so patients treated with sirolimus-eluting stents or other DES were not included. Both cohorts of patients were drawn from the laboratory's computerized database. The cardiac catheterization lab database records the clinical characteristics of patients undergoing hemodynamic study.
Revascularization procedures were performed according to current clinical practice guidelines for percutaneous coronary interventions (PCI).10 The decision to implant a PES was taken by the operator based on a patient's individual clinical characteristics. A loading dose of 300 mg of clopidogrel was administered to all patients who were not already taking the drug. Glycoprotein IIb / IIIa inhibitors were used at the discretion of the operator.
After the intervention, patients received dual antiplatelet therapy (aspirin and clopidogrel) for 6 months if treated with PES and for 1 month in those who received BMS. Afterwards, all patients indefinitely continued monotherapy with aspirin or clopidogrel. This approach contrasts with current recommendations on the duration of dual antiplatelet therapy of 12 months for DES and 1 month for BMS.11 The hospital supplied sufficient doses of clopidogrel to ensure compliance with dual antiplatelet therapy prescribed at discharge.
The primary endpoint was ST. Secondary endpoints were death from cardiovascular causes, non-fatal myocardial infarction, clinical restenosis, and target lesion revascularization.
Medical records and computerized hospital databases were used to describe the study population's baseline characteristics. Major clinical events after the implant were recorded via telephone contact and medical charts and through use of hospital and regional computerized databases. The regional databases record information from both primary and specialized healthcare. The relationship between clinical events and ST was analyzed.
Stent thrombosis was defined according to the current criteria of the Academic Research Consortium,12 and classified as probable or definite. This classification was applied across the range of early, late, and very late ST. Stent thrombosis was defined as probable when there was sudden death from an unknown cause within 30 days after PCI, or when myocardial infarction (MI) was observed in the theoretical area of a previously implanted stent but it was not possible to confirm the presence of a thrombus. Thrombosis was considered demonstrated when stent occlusion by a thrombus was confirmed by angiography or autopsy. Stent thrombosis were classified as early if it occurred within the first 30 days after implantation, late if it occurred between 30 and 365 days after implantation, or very late if it occurred more than one year after implantation.
Acute myocardial infarction (AMI) was defined as cardiac troponin values greater than the 99th percentile of reference values with at least one of the following also present: symptoms consistent with ischemia, Q waves on the electrocardiogram, electrocardiographic changes indicating ischemia (ST-T changes or de novo left bundle branch block), and imaging evidence of loss of viable myocardium or impaired segmental contractility.13
Clinical restenosis was defined as any narrowing of the stent lumen (including the 5 mm proximal and 5 mm distal to the stent) observed after clinical documentation of myocardial ischemia. In the study center, control coronary angiographies are not routinely performed after implantation of a stent.
Statistical Analysis
Continuous variables are reported as means (standard deviation) and categorical variables as absolute frequencies (%). The χ2 test or Fisher's exact test were used to assess the relationship between 2 categorical variables. Means were compared using Student t test or the Mann-Whitney test, depending on the variable's distribution.
The Kaplan-Meier method was used to analyze the long-term incidence of the main study endpoints. The log-rank test was used to compare the time course of events between the 2 study cohorts.
To compensate for the non-randomized nature of the study, analyses were carried out after adjusting by the propensity score,14 ie, the likelihood that each individual be treated according to their clinical characteristics. To calculate each patient's probability of receiving a DES, a logistic regression model was developed in which implantation of a DES was the dependent variable and independent variables were age, diabetes mellitus, previous coronary surgery, renal function, clinical indication, location of injury, primary angioplasty, in-stent restenosis (ISR), bifurcated lesions, and stent diameter and length. The model fit was assessed using the C statistic, which was 0.8 (95% confidence interval [CI], 0.76-0.82).
Adjusted odds for each of the study endpoints were estimated using Cox regression models after adjusting for the propensity score, which was introduced as a covariate in each of the models. To provide separate descriptions of the risk of early and late clinical events, a landmark analysis was performed with a 12 month default time limit.15
Statistical analyses were performed in SPSS (Statistical Package for Social Sciences) version 17.0 for Windows. A result was considered statistically significant if P<.05.
RESULTS
A total of 1268 patients were included in the BMS cohort and 430 in the PES cohort. Follow-up was completed for 1674 patients or 98% of those originally included (422 patients in the PES group and 1252 in the BMS group). Only 24 patients were lost to follow-up, but no clinical event was registered for those patients in healthcare databases and, based on official data, all were alive at the end of the study.
The baseline characteristics of the 2 groups are shown in Table 1. Compared with patients who received BMS, those treated with PES were younger and more often diabetic. In the PES group, patients more frequently had a history of prior percutaneous or surgical revascularization. Multi-vessel disease, disease of the anterior descending coronary artery and left main coronary artery, treatment of ISR or bifurcated lesions were also more frequent in the PES group. Average stent diameter was significantly lower in the PES group and covered stent length was significantly greater. Primary angioplasty was more frequent in the BMS cohort, which also had worse left ventricular function than the PES cohort.
Stent Thrombosis
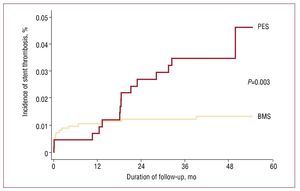
After a median follow up of 46.1 [interquartile range, 13] months, the incidence of probable or definite ST was higher in the PES group than in the BMS group (3.5% vs 1.3%; P=.003). There were no differences in the incidence of early (0.5% vs 0.8%; P=.574) or late ST (0.23% vs 0.32%; P=.769). However, very late ST was more frequent in the PES group than in the BMS cohort (2.8% vs 0.24%; P<.001) (Figure, Tables 2 and 3).
Figure 1. Incidence of stent thrombosis in paclitaxel-eluting stents (PES) and bare metal stents (BMS).
After propensity score adjustment, the risk of ST was higher in PES patients than in the BMS group (hazard ratio [HR] =3; 95% confidence intervals [CI], 1.2-7.1; P=.014). No differences in risk adjusted ST were observed during the first year after the index PCI (HR=0.8; 95% CI, 0.2-3.4; P=.767), while PESs were associated with an additional risk of very late ST compared to BMSs (HR=12.8; 95% CI, 3-55.1; P=.001) (Table 4).
The ST presented as AMI in all cases except 2 (2 sudden deaths in the first 30 days after PCI were classified as probable ST, with 1 case per group).
Cardiovascular Mortality
There were no differences in cardiovascular mortality between the PES and BMS groups during follow-up (5.3% vs 7.3%; P=.26), either during the first year of follow-up (2.6% vs 2.7%; P=.868) or after (2.8% vs 4.6%, P=.186) (Table 3).
After propensity score adjustment, there were no significant differences in the risk of cardiovascular death between the BMS and PES groups (HR=0.7; 95% CI, 0.4-1.2; P=.195) either during the first year (HR=1.1; 95% CI, 0.5-2.4; P=.876) or after (HR=0.6; 95% CI, 0.3-1.2; P=.122) (Table 4).
Non-Fatal Acute Myocardial Infarction
The incidence of AMI was higher in the PES group than in BMS group, though the difference was not statistically significant (7.4% vs 4.2%; P=.277). This event was more frequent in PES patients from the first year on, although again the difference was not statistically significant (5.6% vs 1.8%, P=.175). In the first 12 months there were no differences between the 2 groups (2.4% vs 1.9%, P=.513) (Table 3).
During follow-up, there were no significant differences in risk-adjusted non-fatal AMI between the PES and BMS groups (HR=0.7; 95% CI, 0.4-1.3; P=.215). When the analysis was performed for specific time periods, there was no difference during the first 12 months of follow-up (HR=0.6; 95% CI, 0.3-1.3; P=.16), but beyond 12 months PESs were associated with a marginally increased risk of AMI compared to BMSs (HR=2.6; 95% CI, 0.9-7.7; P=.089) (Table 4).
Stent Restenosis
Stent restenosis was less frequent in the PES group (cumulative incidence of 2.8% vs 5.4% in the BMS group; P=.025). This difference was primarily seen during the first year of follow-up (2.1% vs 4.1%; P=.05), with no statistically significant differences observed beyond that point (0.7% vs 1.3%; P=.291) (Table 3).
The use of PES reduced the risk-adjusted likelihood of restenosis compared to BMS (HR=0.3; 95% CI, 0.2-0.7; P=.001), particularly in the first 12 months (HR=0.32; 95% CI, 0.2-0.7; P=.003). Beyond that point, the difference was not statistically significant (HR=0.4; 95% CI, 0.1-1.6; P=.206) (Table 4).
Target-Lesion Revascularization
There were no statistically significant differences in the incidence of target lesion revascularization (TLR) between the PES and BMS groups (5.1% vs 6.5%; P=.282). In the first year, the incidence of TLR was lower in the PES group (2.3% vs. 5%; P=.017); from that point on, a higher incidence of TLR was observed in the PES group, with the difference approaching statistical significance (2.8% vs 1.5%; P=.084) (Table 3).
There were no differences in risk-adjusted TLR between the 2 devices (HR=0.8; 95% CI, 0.5-1.3; P=.355). However, the risk of TLR in the first year was significantly lower in the PES group (HR=0.33; 95% CI, 0.2-0.7; P=.002). After 12 months, PES showed an excess risk of TLR compared with BMS (HR=1.8; 95% CI, 1-3.2; P=.05) (Table 4).
DISCUSSION
Late thrombosis is the main concern with PESs.16,17 The main finding of this study was that an excess risk of ST with PES compared to BMS could limit the clinical benefits obtained by a lower risk of restenosis with DESs. The risk of ST with PESs was greater in absolute terms in this real-life scenario than in experimental situations.
In this study, PES was associated with an excess risk for ST compared to BMS, due to very late ST. The incidence of ST with PES in this series (3.5%) was higher than that reported in several meta-analyses.3-8 This is probably due to the more complex clinical situation of the patients in our sample. In fact, this factor has been identified as a serious drawback of meta-analyses in quantifying the impact of late ST with DESs.9 Other observational studies have reported varying figures, but they have generally been significantly lower than those reported here (generally <1%).18,19 On the other hand, they have usually used shorter follow-up periods (<2 years). As DES are consistently associated with late ST,20, 21 the duration of our follow-up likely explains the difference between our findings and those of previous studies. Cumulative incidence at 1 year in our PES group (0.5%) was in fact similar to that reported in previous studies. In addition, most of the ST in the PES group were very late ST (12 [80%] of 15 cases); these events would not have been recorded in studies with shorter follow-up periods. On the other hand, the incidence of ST with PES in our study was consistent with rates reported by other, more recent studies with similar follow-up periods.22
Our study supports the existence of a risk-benefit ratio for PES which varies over time, and which differs from BMS in terms of the incidence of early and late ST.
The use of PES did not significantly reduce the need for new revascularization procedures compared with BMS. However, during the first year of monitoring, PES markedly reduced the need for TLR, and reduced the risk of ISR. However, thereafter, the use of SLP was associated with a 13 times greater risk of ST, which resulted in an increased risk of TLR compared with BMS. Similarly, there was no difference in the rate of revascularization of the treated vessel (TVR) at 3 years between DESs and BMSs in the BASKET LATE study (TVR rate of 14.7% for DES compared to 17.5% for BMS; P=.29), a fact which the authors linked to ST.23 In randomized studies, DESs reduced the need for new revascularization procedures compared with BMS.1,2 These studies underestimate the incidence of late and very late ST because they systematically exclude clinically complex patients; this in turn may mean they overestimated the benefit of DESs by minimizing the impact of late ST. Other observational studies have also shown a decrease in the need for further revascularization with DES compared to BMS, but they also tend to underestimate the volume of ST in DES because of the use of short follow-up periods.24-26
The role of ISR in the absence of statistically significant differences in terms of TLR between the PES and BMS groups deserves comment. We found the risk of ISR to be markedly reduced with PES, a finding which conforms with the large body of evidence available today. However, the low rate of ISR (5.8%) after implantation of a BMS is striking, and we found little difference in absolute values as regards the need for TLR in BMS and PES patients (1.4%). Both rates are lower than those reported by other studies, although the SCAAR register recorded similar figures.15 By using a clinical criterion to define ISR, it is likely that we underestimated its impact and this may have contributed in part to the lower observed benefits of DES in comparison to other studies performed in populations in which an increased risk of restenosis would be expected.
Because of the modest sample size, our study lacked statistical power to detect significant differences in TLR rates between the 2 devices. However, if we take into account that the development of very late ST is not well understood and that continued occurrence of extraordinarily late ST (several years after implantation) cannot be ruled out, then further studies with longer follow-ups are required to assess the possible clinical impact of TLR and its importance in clinical comparisons of BMS and PES.
No differences in risk-adjusted non-fatal AMI were observed between PES and BMS. During the first year, PESs showed a tendency to decrease the risk of AMI compared with BMS, a reduction related to the reduction in ISR. Thereafter, however, this trend was reversed, leading to a tendency towards increased risk of AMI with PES. This increase almost achieved statistical significance and was clearly related to the excess risk of very late ST presented by these devices.
This crossover phenomenon has been reported recently by other authors. In the BASKET LATE study,23 DESs did not increase the risk of death or AMI at 3 years compared with BMSs. However, mortality rates and AMI were higher in DES patients than in BMS patients after 6 months, a rise which paralleled the excess of ST. The SCAAR register15 also showed that adjusted risk of myocardial infarction was lower in DESs at 6 months. Thereafter, there was a non-significant trend toward an increased risk of myocardial infarction with these stents. In the landmark analysis, the curves for AMI were separated in time, especially after the first 12 months. In the Ontario study,26 the rate of myocardial infarction at 2 years was similar in the DES and BMS groups. However, the rate was 0.4% lower with DES compared to BMS during the first six months. After 15 months, a crossover phenomenon was observed, whereby the rate was 0.5% higher in DES than in BMS. Follow-up data for these last 2 studies were obtained from administrative databases, so it was not possible to quantify the incidence of ST and to assess the probable relationship between very late ST and an excess risk of events after the first year with DES. From our perspective, the very late ST observed with DES is a reasonable explanation for this crossover phenomenon, although selection bias cannot be excluded.
Study Limitations
This study may have several limitations. Data were collected from a registry in a single institution so the study may not be free of the biases inherent to this type of design. Patient follow-up was not complete. Patients who are lost to follow-up tend to have a greater number of events, which could modify the study results. Our results should be interpreted with caution, as confounding variables may have contributed to differences after adjustment. Since we included only patients with PES, these results may not be applicable to other types of DES. In fact, PESs appear to entail greater risk of ST than other DES.27 A further limitation was that compliance with dual antiplatelet therapy was not monitored during follow-up. Finally, these findings derive from usual practice in our cardiac catheterization lab and might not be reproducible in labs with a different policy of DES implantation.
CONCLUSIONS
Under clinical practice conditions at the study center, PESs were associated with increased adjusted risk of ST compared to BMSs. The difference was primarily due to excess risk of very late ST with PESs. Our findings indicate the existence of a differential risk-benefit ratio in the early and late stages after implantation of a PES. With PESs, the initial benefit obtained from a reduction of ISR and of the need for further revascularization is offset by the excess risk of very late ST. When compared to BMS, very late ST in DESs appears to limit the clinical benefits achieved through a reduction in new revascularization procedures related to a decline in ISR. The results should be interpreted with caution due to the non-randomized nature of the study. Confounding factors may also contribute to differences after adjustment. Further studies are required into the evolution of very late ST and its clinical consequences.
ABBREVIATIONS
AMI: acute myocardial infarction
BMS: bare metal stents
DES: drug-eluting stents
HR: hazard ratio
ISR: in-stent restenosis
ST: stent thrombosis
TLR: target lesion revascularization
Correspondence: Dr. X. Flores Ríos.
Servicio de Cardiología. Complexo Hospitalario Universitario A Coruña. As Xubias, s/n. 15006 A Coruña. España.
E-mail: xacobeflores@yahoo.es
Received September 13, 2009.
Accepted for publication December 16, 2009.