Keywords
INTRODUCTION
A disparity has been recognized between the recommendations found in clinical guidelines and daily clinical practice which is reflected in different registries regarding the management of ischemic heart disease. There are many examples of this both within Spain and at international levels, some being very recent, in patients with acute myocardial infarction (AMI) or acute coronary syndromes.1-8 Such differences have been found in acute management as well as in risk stratification and secondary prevention strategies.2,9,10
Although knowledge concerning this disparity could theoretically lead to improvement, practice shows that this is not always the case, at least, not substantially.11,12 This has led to developing strategies aimed at improving care in patients with ischemic heart disease. These include the identification of quality indicators, their correct dissemination, and the development of new tools to help reach the agreed quality standards.
Currently, there is interest regarding the issue of quality in the areas of general medicine and cardiology.13-17 Much of this interest is due to the increasing complexity of medicine and the appearance of new technologies for the distribution of medical information which has led to demands for continuous improvement on behalf of the society.18
Similar approaches have not been followed in Spain to date, except in very local instances. Thus, the aim of this study was to evaluate the effectiveness of a mainly educational intervention program for professionals dealing with different aspects to the approach and treatment of acute coronary syndromes with or without ST segment elevation.
PATIENTS AND METHOD
General Design of the Study
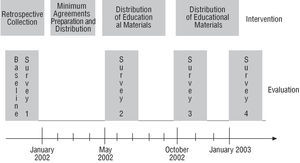
The study was done in 39 Spanish hospitals during 2002 and 2003. In the participating centers, follow-up was carried out on the effect of a simple intervention to improve care in patients with acute coronary syndrome. A general overview of the project is outlined in Figure1. The hospitals in the sample were selected according to convenience and not randomly. The geographical distribution of the participating centers is presented in Figure 2.
Fig. 1. General overview of the project.
Fig. 2. Geographic distribution of the participating centers.
Description of the Intervention
1. The development, by consensus in all centers, of a set of agreed minimums (Table 1) covering all the basic aspects of intervention in these patients. The quality indicators to be evaluated16 (Table 2) derive from this agreement.
2. Preparation of materials to facilitate adherence with the agreed minimums: posters and leaflets outlining the agreed minimums and the aspects that should be improved, as well as a CD containing the presentation of the project and the general and specific results from every center.
3. The materials were disseminated by distributing the leaflets among all the physicians from a participating service and those related to it (mainly internal medicine), by putting up the posters in areas where patient discharge reports are made out, and via local meetings set up to discuss and promote the project in every center.
Evaluation of the Intervention
Four cross-sectional surveys were carried out. In each survey, every hospital had to select 30 consecutive patients with a diagnosis of acute coronary syndrome (unstable angina or AMI with or without ST segment elevation)19 discharged alive by the participating service. In the first survey, information collection was carried out retrospectively, corresponding to a date prior to the beginning of the project, to avoid the Hawthorne effect (improvement due to knowing one is observed). In the remaining surveys, the information was collected prospectively based on the date indicated. The data sources in all cases were the discharge report and the medical record. The surveys were carried out at different times that coincided with the various survey stages: a) the first survey was done before the beginning of the study; b) the second survey was done after approving the agreed minimums; and c) the third and fourth surveys were carried out after distributing the intervention materials; the third soon after distribution began and the fourth around 4 months later (Figure 1). The final sample was made up of 4626 patients (1157, 1162, 1149, and 1158 in surveys 1 to 4, respectively).
An external review of the quality of the data collected was not carried out. The database was reviewed to detect and correct typographical errors, anomalous values or inconsistencies in the data.
The "Ideal Patient" Concept
These are patients who, according to the agreed minimum, should receive a specific instruction or procedure. The percentages presented frequently refer to these patients. This datum is interpreted as the percentage of patients who actually receive treatment or exploration compared to the total of those who should receive it according to the agreed minimums.
Statistical Analysis
A descriptive analysis was carried out by means of the frequency distribution of qualitative variables, the calculation of percentages for binary variables, and the calculation of standard measurements (mean, standard deviation, minimum, and maximum) for continuous variables. Confidence intervals (95% CI) were obtained for the percentages and means.
The Levene test for homogeneity of variance was done to compare the variability of the percentages by hospital between the 4 surveys and, in the event of significant differences, a linear trend analysis of variance over time was carried out.
The global percentages of the 4 cross-sectional surveys were compared through a 2-factor analysis of variance (survey and hospital), for which the transformation recommended for binomial data was previously carried out (arcsin√p, where p represents each percentage).20 As in the previous case, when significant differences were found a linear trend analysis of the percentages was carried out at a 5% significance level.
RESULTS
A total of 4626 patients in the 39 participating centers were included during the study period. At discharge, 47.6% of patients had a diagnosis of AMI with ST segment elevation, 27.8% had AMI without ST segment elevation, and 24.6% had unstable angina.
Demographic and Clinical Characteristics at Admission
The baseline characteristics of the patients analyzed in each cross-sectional survey are presented in Table 3. No differences were observed in the demographic and clinical characteristics of the patients included in each survey.
Quality Indicators at Admission
The measurement of lipids at admission, independently of when this was done, reached 87.6% in the preintervention survey and increased slightly to 92.1% in the final survey. When analyzing the percentage of samples taken within the first 24 h, an increase was found ranging from 42.6% in the preintervention survey up to 56.5%, 56.6%, and 53.7% in the 3 later surveys, respectively (Table 4).
The criterion for weight and height was not fulfilled in 66.5% of cases in the baseline survey, decreasing to 46.7% in the final survey (Table 4).
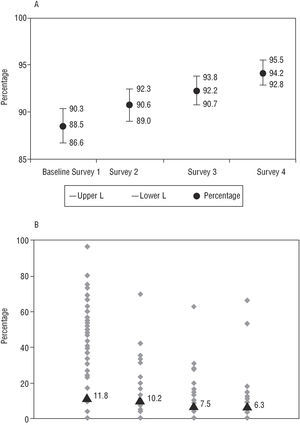
Regarding the criteria for stratifying risk in the patients analyzed, an absolute improvement of 3.3% was found in the indicator for evaluation of ejection fraction (EF) when only ideal patients were considered (those in whom this should be carried out according to the agreed minimums) (Table 4) and of 5.7% in the group of patients as a whole (Figure 3). The measurement of residual ischemia did not comply with the agreed minimums in the baseline survey in 18.9% of the patients; this decreased to 10.8% in the final survey (a relative improvement of 43% regarding this criterion). Of the total patients, residual ischemia was not measured in 11.8%, 10.2%, 7.5%, and 6.3% of patients in each survey, respectively (Figure 3). In both tests the variability of the indicator decreased linearly between the centers from the time of the preintervention survey (P<.05). At the same time, a clear growing linear trend was observed in the global indicator (P<.05).
Fig. 3. Risk stratification indicators. A: Measurement of ejection fraction in the group of patients. B: Measurement of residual ischemia in the group of patients (percentage of patients in whom this was not carried out).
Quality Indicators for Pharmacological Prescription
The administration of acetylsalicylic acid (ASA) in the group of patients varied slightly between the different surveys (89.2%, 87.0%, 89.9%, and 91.7% in the baseline, second, third, and fourth surveys, respectively). Appropriate use of platelet aggregation inhibitors at the time of discharge varied slightly, although statistically significantly, from the baseline survey where a very high level of adherence (95.9%) (Table 4) was already observed. The use of nitrates was not justified in the baseline survey in 12.1% of cases, a quality indicator that improved gradually to 8.1% in the final survey, but without a clear linear trend (Table 4). A linear trend toward improvement was in fact found in the case of calcium antagonists.
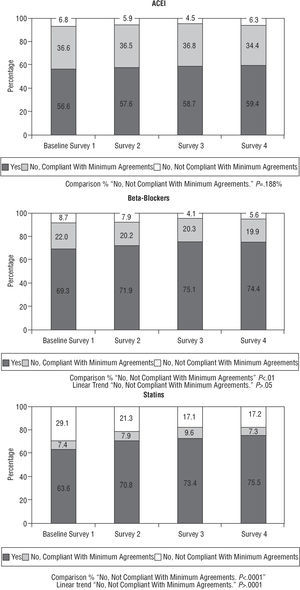
Figure 4 shows the percentage of patients who were prescribed angiotensin-conversion enzyme inhibitors (ACE inhibitors), beta-blockers (BB) and statins, as well as the percentage of those who were not prescribed any of these for no apparent reason. This information is complemented in Table 4 by the estimation of the percentage of patients who received such prescriptions versus those who should have (ideal patients).
Fig. 4. Pharmacological treatment at the time of discharge.
In the baseline survey, 68.6% of the patients who should have received statins as agreed did in fact receive them. This percentage improved in successive surveys up to 81.4% in the final survey, with an 12.8% absolute improvement in the indicator and 19% in relative terms.
Regarding prescriptions for BB, their use improved compared to the initial measurement, with a slight upturn in their justified non-prescription in the final survey (8.7%, 7.9%, 4.1% and 5.6%, respectively) (decreasing linear trend, P<.05).
Prescriptions for ACE inhibitors presented a similar pattern, with progressive improvements in the second and third surveys (5.9% and 4.5%, respectively) with regard to baseline (6.8%) and another increase in their underprescription in the fourth (6.3%) (Table 4 and Figure 4).
Quality Indicators Referring to the Recommendations Included in the Discharge Report
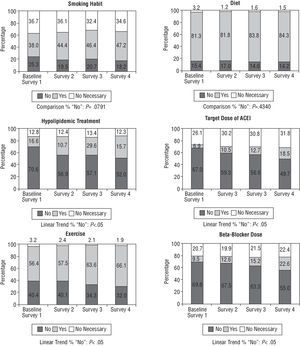
The distribution of participants is presented in Figure 5 regarding the different recommendations agreed upon in the discharge report. This information is complemented in Table 4 by using the ideal patients as common denominators in each case. Anti-smoking advice was not given in 39.9% of patients who smoked in the baseline survey, although this indicator improved in the following surveys (30.5%, 30.6%, and 27.8%, respectively). Advice concerning diet was not given when indicated to 15.9%, 17.2%, 14.8%, and 14.4% of patients, respectively.
Fig. 5. Treatment at the time of discharge.
No recommendations regarding exercise were given in 41% of the cases in the preintervention and second surveys, although this indicator improved in the final survey compared to the previous two (35% and 32.6%, respectively) (statistically significant linear trend, P=.003).
The target levels and doses of lipid-lowering drugs and the target doses of ACE inhibitors and BB in patients for whom they were indicated greatly improved in each survey.
DISCUSSION
The CAM survey data demonstrate that it is possible to improve the quality of care in patients hospitalized for acute coronary syndromes in a multicenter context, with significant improvements in the procedures carried out, the pharmacological therapeutic measures, and the recommendations at the time of discharge. This improvement was obtained after the application of an educational intervention together with the measurement of and feedback on the data evaluated in each center.
Multiple registries are available on the management and treatment of patients with acute coronary syndromes.3-11 The methodology used and the value of the information obtained have been subject to criticism.21 However, this current project differs from a conventional registry in several respects. First, it provides estimations of the percentage of patients who receive a given treatment (or other measures) out of those who should receive it, and not just out of the total number of patients as found, with few exceptions, in the available registries. This was done with the prior explicit written agreement of all the centers involved in regard to the interventions that should be carried out. Second, an intervention common to all the participating hospitals was done with a later reassessment to evaluate its potential benefits; this is not the aim of a registry and thus is not available in any Spanish registry. There are similar studies on different intervention techniques in the international or Spanish context, but none of these are multicenter studies.22-24 Such intervention strategies aimed at improving quality have shown their efficacy in patients with AMI, both locally and at a multicenter level. The interest raised by quality issues in cardiology has led the American College of Cardiology and the American Heart Association to develop quality improvement programs for AMI with more complex tools that have demonstrated their applicability and efficacy, such as the Guidelines Applied in Practice and Get With the Guidelines projects, respectively.25,26 These strategies have also demonstrated their efficacy in other areas in patients with ischemic heart disease, such as lipid management or coronary surgery.27,28 In Spain, the results of the PRESENTE survey, an intervention program for postinfarction patients and their families, have recently been published showing significant improvements in secondary prevention measures. This survey dealt with a different popul ation and intervention, but whose initial aim was to improve quality of care via intervention programs.29
Analysis of Improvement
All the quality indicators related to hospital management (analysis of cholesterol, weight and height, and measurement of EF and ischemia) improved after the intervention, with gradual positive increases in the three postintervention surveys. The most striking improvements occurred in the measurement of the lipid profile within the first 24 h (an absolute increase of 9.8 points) and in the measurement of weight and height at admission (an absolute increase of 22 points). Obviously, these match those areas with a greater chance of improvement at baseline. However, absolute improvements of 5.5% should not be ignored in the correct assessment of ischemia before discharge or of 5.7% in the evaluation of ventricular function.
Regarding pharmacological prescription at the time of discharge, if the indications for a drug are analyzed without assessing whether it was prescribed for an "ideal" patient, the figures are similar to the prescription for ASA (89.2%-91.7% in the CAM study vs 87.8% in the PREVESE II12 study or 93% in the PEPA5 study). The figures diverge regarding the use of ACE inhibitors, BB and statins, these being greater in our survey (56.6% ACE inhibitors, 69.3% BB, and 63.6% statins at baseline vs the PREVESE II data, with 46.4% ACE inhibitors, 45.1% BB, and 29.4% statins, or vs the PEPA data, with 20% ACE inhibitors, 42% BB, and 6% statins). First, these differences can be accounted for by the fact that in the CAM survey acute coronary syndromes with and without ST segment elevation were analyzed jointly; second, by the amount of time that had elapsed since those registries were created since awareness about and evidence of the use of secondary prevention measures is currently greater.
When the "ideal" patients are actually analyzed (those who did not have any absolute or relative contraindications for the indicator under analysis), our results can only be compared with those of the Guidelines Applied in Practice project.25 This survey was carried out in patients with AMI in which a different intervention tool was used. A change was found from 89% preintervention to 93% postintervention in prescriptions for BB, from 80% to 86% for ACE inhibitors, and from 68% to 75% for statins. In our survey there was a change (when comparing the baseline to the fourth survey figures) in prescriptions for BB in ideal patients from 88.9% to 92.9% (fourth survey); from 89.3% to 90.4% for ACE inhibitors; and from 68.6% to 81.4% for statins (Table 4). These data are similar, except in the ACE inhibitors group, probably because the indication level was set higher in our case and thus there was a smaller chance for improvement.
There was an improvement after the intervention regarding the recommendations at the time of discharge on smoking, diet, exercise, or target dose of drugs (ACE inhibitors or BB), although this was variable (from 1.5 improvement points in stating appropriate recommendations for diet, with nonsignificant differences, up to 21.6 improvement points regarding stating the target levels of low-density lipoprotein cholesterol (LDL-C) in the discharge report). It should be pointed out that, regarding basic aspects such as diet or exercise unrelated to pharmacological treatment, smaller improvements were obtained. One possible way to read this is that the interventions used in this survey which lead to changes in lifestyle are not as effective as the pharmacological ones. It may be necessary to carry out structural and multidisciplinary changes in patient care and, in the longer term, with the more active involvement of other professionals (nurses and others).
Most of the observed improvements were accompanied by or were due to a reduction in variability between centers, which constitutes, in our opinion, the added value of the multicenter approach.
Limitations
This study has several limitations. First, at the time of publication, we lacked a control group of hospitals with which to compare the efficacy of the intervention. Due to this, it cannot be completely ruled out that some of the observed improvements may have also occurred in the absence of any intervention. Nevertheless, this seems unlikely, especially regarding the measures that have been established for a long time (e.g., the measurement of cholesterol within the first 24 h, prescriptions for BB at discharge, etc). Second, the participating centers were not selected randomly. The process of assignment to the survey was voluntary and, therefore, this could already imply concern for improvement in these centers. This might partly explain the good baseline situation. However, this could not be the case in centers without a suitable level of motivation. In brief, and if this is correct, improvement will occur only if there is a desire to improve, which seems reasonable. In addition, there was no external control to guarantee the objectivity of data collection. The probability of the deliberate alteration of data collection seems low since, given the design of the survey, confidentiality was maintained regarding the results of each center. Thus, modifying the data would give any such center a distorted picture of its own situation, without being counterbalanced by the recognition of a job well done. The most likely situation is that, as it was known that the intervention was being monitored, certain practices would be partially changed. However, this is not regarded as a problem, but as a desirable outcome in the context of a program designed to continually improve quality.
Attention should be drawn to the apparent decrease in some of the quality indicators in the final survey compared to the two previous surveys, which raises the question of whether this can be attributed to a loss of motivation once the first phases had ended. If so, more reliable tools or structural strategies need to be constructed to avoid this situation.
This project is innovative in that it is the first to approach improvement in quality for patients with ischemic heart disease in a multicenter context. Whether this intervention can be improved or not, or whether it persists or not, is debatable. However, it does provide clear benefits regarding the proposed quality indicators based on this new methodology, although this is still being developed. On the other hand, this survey is also novel because it provides estimations regarding the patients who should receive each treatment or intervention. In our opinion, this provides a more realistic vision of the magnitude of the gap between what is done and what should be done, as well as a better idea of how much improvement is possible.
In conclusion, our survey shows that the application of an intervention based on quality indicators can improve the quality of care in patients with ischemic heart disease in a multicenter context.
CENTERS AND RESEARCHERS PARTICIPATING IN THE CAM PROJECT ("THE SOONER THE BETTER" "CUANTO ANTES MEJOR") CENTER COORDINATOR: ODDS, S.L., LA CORUÑA (MARIA I. SANTIAGO PÉREZ).
Management Committee: Alfonso Castro Beiras, José María Cruz Fernández, Javier Muñiz García, and Eduardo de Teresa Galván.
Development Committee for Agreed Minimums: Enrique de los Arcos Lage, Juan José Gómez Doblas, Iñaki Lekuona Goya, Javier Muñiz García, Vicente Nieto Lago, José Ángel Rodríguez Fernández, and José Velasco Rami.
Editorial Committee: Alfonso Castro Beiras, José María Cruz Fernández, Juan José Gómez Doblas, Javier Muñiz García, and Eduardo de Teresa Galván.
Alicante: Hospital General Universitario de Alicante (José Cánovas Robles). Asturias: Hospital Central de Asturias (Vicente Barriales Álvarez). Badajoz: Hospital Universitario Infanta Cristina (Juan J. García Guerrero). Baleares: Hospital Son Dureta (Miguel Fiol Sala and Andrés Carrillo López). Barcelona: Ciutat Sanitària i Universitària de Bellvitge (Fernando Worner Diz). Hospital Clínic i Provincial de Barcelona (Antonio Francino Battle). Corporació Sanitària Parc Taulí de Sabadell (Ignasi Angera). Hospital de Terrassa (Ali Jaber Houbani). Cádiz: Hospital del SAS de Jérez (Jesús Martín Miranda). Cantabria: Hospital Universitario Marqués de Valdecilla (Jesús Gutiérrez Morlote). Córdoba: Hospital Universitario Reina Sofía (José M. Arizón del Prado and Natalio Martín Montes). A Coruña: Complejo Hospitalario Universitario Juan Canalejo (José A. Rodríguez Fernández). Complejo Hospitalario Universitario de Santiago de Compostela (Michel Jacquet). Girona: Hospital Universitario de Girona Dr. Josep Trueta (Isabella Rohlfs Barroso). Granada: Hospital Virgen de las Nieves (Manuel García Delgado). León: Hospital de León (Nuria Marcos González). Madrid: Fundación Jiménez Díaz (José L. Romero García). Hospital General Universitario Gregorio Marañón (Rafael Rubio Sanz). Hospital Universitario La Paz (Juan L. Martín Jadraque and Eduardo Armada Romero). Hospital Universitario 12 de Octubre (Jesús Rodríguez). Hospital Clínico Universitario San Carlos (Juan C. García Rubira). Málaga: Hospital Clínico Universitario de Málaga (Juan J. Gómez Doblas). Murcia: Hospital General de Área Santa María del Rosell (Francisco Jiménez Pagán and Juan Martínez Hernández). Navarra: Hospital Virgen del Camino (Irene Madariaga Arnaiz). Hospital de Navarra (Enrique de los Arcos Lage). Las Palmas: Hospital Universitario Insular de Gran Canaria (Gloria O'Shanahan Navarro and Vicente Nieto Lago). Pontevedra: Complejo Hospitalario de Pontevedra (Enrique Iglesias Río). Santa Cruz de Tenerife: Hospital Universitario de Canarias (Alberto Domínguez Rodríguez, Martín García González, and Antonio Barragán Acea). Sevilla: Hospital Universitario Virgen del Rocío (Antonio Puppo Moreno). Hospital Clínico Universitario Virgen Macarena (Pastora Gallego García de Vinuesa and Manuel Almendro Delia). Toledo: Hospital Nuestra Señora del Prado (Almudena Simón Martín and Manuel Quintana Díaz). Valencia: Hospital Universitario La Fe (José L. Marqués de Fez and Adolfo Cabades O'Callaghan). Hospital General Universitario de Valencia (José Velasco Rami e Ildefonso Echanove Errazti). Hospital Clínico Universitario (Rafael Sanjuán and Marisa Blasco). Valladolid: Hospital Clínico del Río Hortega (Juan J. Sanz Hernán). Vizcaya: Hospital de Cruces (Jenaro Froufe). Hospital de Galdácano (Iñaki Lekuona Goya). Zaragoza: Hospital Miguel Servet (Carlos Vázquez Rodríguez and Cristina García Laborda). Hospital Clínico Universitario Lozano Blesa (José Luis del Río Aiza and Alfonso del Río Ligorit).
*The centers and researcherlved in the CAM Project are listed at the end of the article. The CAM Project was made possible thanks to unconditional help from Merck Sharp and Dohme España, who approved the design proposed by the researchers but did not participate in any phase of the project, including data analysis and the preparation or review of this article.
Correspondence: Dr. J. Muñiz García.
Instituto Universitario de Ciencias de la Salud.
Hospital Marítimo de Oza, pabellón n.o 6.
As Xubias, s/n. 15006 A Coruña. España.
E-mail: javmu@udc.es