Keywords
INTRODUCTION
Adenosine is an endogenous nucleotide composed of adenine and a pentose sugar. It is the product of several metabolic pathways. Many of its actions have been known for years. These include cardiovascular effects such as negative inotropism and chronotropism, vasodilation, and modification of conduction, as well as extra-cardiovascular effects such as the release of neurotransmitters, central nervous system activity, and the modification of platelet and leukocyte functions, lipolysis, and glucose metabolism. Not all the effects of adenosine are known, however, since the molecule also acts at the intracellular level. In cardiac cells, adenosine acts via four different G protein-coupled sarcolemmal receptors: A1, A2A, A2B, and A3.1 G proteins, which differ in their amino acid sequence and molecular weight, regulate the effects caused by the stimulation of these receptors, and activate both K+ and Ca2+ channels. They may also activate phospholipase C-β.2 G protein-coupled receptor A1 reduces cyclic adenosine monophosphate (cAMP) concentrations by antagonizing the activity of adenyl cyclase.2 A number of other cardiac cell adenosine receptors are known, some of which influence muscarinic potassium channels (KACh, KADO). These receptors mediate the chronotropic and dromotropic effects of adenosine in the atria, but not in the ventricles. When adenosine accumulates in the extracellular spaces, the outflow of K+ increases, provoking a reduction in the conduction velocity of the atrioventricular cells.3,4
In ventricular myocardial cells, receptor A1 appears to be associated only with the inhibition of adenyl cyclase (via inhibitory G proteins), which explains why adenosine attenuates the stimulatory effects of catecholamines on beta-adrenergic receptors.5 The drug represses the automatism of sinoatrial cells as a result of cellular hyperpolarization, and therefore reduces the discharge frequency of the sinus node. Thus, adenosine provokes the blockage of impulses from this node, and reduces the automatism and conduction velocity of the atrial cells and the atrioventricular node.3,4 At the ventricular level its action is indirect, and therefore can be effective in problems related to the increase of catecholamine concentrations.6-8
The clinical use of adenosine in the treatment of supraventricular arrhythmias has been a great success and is widely accepted. It has also been used for the differential diagnosis of tachyarrhythmias with aberrant QRS complexes (this has to be done carefully, however, since adenosine can facilitate conduction via the anomalous route if there is pre-excitation, leading to ventricular fibrillation [VF]).9,10 The rapid onset of action of adenosine plus its short half life have made its use common in these areas.
The effectiveness of adenosine in the treatment of supraventricular arrhythmias is similar to that of verapamil and is associated with fewer secondary effects.11 However little has been published on its use in the treatment of ventricular tachyarrhythmias. Animal and human studies performed to date have shown that adenosine has no direct electrophysiological effect on the ventricular myocardial fibers nor on the His-Purkinje system.7 Its indirect effect is related to its antagonizing increases in cAMP concentrations, mediated by the inhibition of adenyl cyclase.12 Adenosine is not considered useful in the treatment of ventricular arrhythmias of ischemic origin since it does not directly affect transmembrane action potentials.13 However, it is effective in idiopathic ventricular tachycardia, and in tachycardia in structurally normal hearts-problems that appear to be related to triggered potentials14 only when the adrenergic system is involved and late post-potentials are generated (phase 4 of the transmembrane action potential). Finally, intravenously administered adenosine can induce a sympathetic reflex leading to sinus tachycardia, atrial or ventricular extrasystole, non-sustained ventricular tachycardia, and atrial fibrillation (AF).11,15,16
We have previously reported on the favorable effects of adenosine in some cases of ventricular tachycardia (VT) probably induced by triggered potentials.17 The present paper reports the effects of this agent on experimental VT due to triggered potentials induced by aconitine.18 The action of adenosine is also described in VT probably maintained by micro-reentries formed at the periphery of small areas of myocardial damage. In the present work, this damage was induced by concentrated phenol in order to obtain well defined lesions--such limited damage cannot be achieved by coronary artery ligature. Both early and delayed anti-arrhythmic effects were recorded. It is proposed that these early effects are due to adenosine itself (it should be remembered that the half life of this agent is short), and that the delayed effects are due to a catabolite of adenosine, perhaps inosine, previously considered inactive.19
METHODS AND MATERIALS
The anti-arrhythmic action of adenosine (the fleeting or transitory restoration of sinus rhythm [SR]) was studied in 173 mongrel dogs weighing between 13 and 17 kg. Sixteen animals were also used in preliminary studies.
All animals were anesthetized with 30 mg/kg intravenous sodium pentobarbital, connected to an artificial respiration system, and received continuous infusion of Hartmann solution. After opening the thorax to expose the heart (open pericardium), 1 mL-1.5 mL of 70% phenol (depending on the weight of the animal) were injected into the myocardium close to the apex of the left ventricle. This produced a circumscript, well defined myocardial lesion. Some 15 to 30 minutes later, VT (generally left) was induced by introducing small crystals of aconitine (Sigma) into the myocardium next to the damaged area. Some 15 to 30 minutes later, an adenosine bolus (see below) was injected into the superior vena cava. Sixteen dogs were previously tested to confirm that adenosine had an anti-arrhythmic effect on VT. A control group of 72 dogs underwent the above procedure but received no adenosine bolus. In the remaining 85 animals, the early and delayed effects of 6 mg and 12 mg of adenosine sulfate (Sigma) were recorded. VT was induced by aconitine in 63 dogs. Of these, 20 received a single injection of 6 mg (group A), 21 received the same dose again 60 min later (group B), 12 received 12 mg of adenosine as a single dose (group C), and the remaining 10 received this dose twice (group D). The same doses were also given to other 18 dogs (6 mg to 6 and 12 mg to 12 animals) whose ventricular arrhythmia were due exclusively to the myocardial damage induced (i.e., no aconitine was administered). Only in 6 of the 12 animals that received the 12 mg dose was it possible to inject a second, equal dose. This same dose (2x12 mg) was also administered to four animals who showed spontaneous VT, i.e., before administering the phenol and aconitine.
In all experiments, readings were taken from leads DII, aVR or aVL, from the unipolar right and left intraventricular leads, and from one unipolar lead on the wall of the superior vena cava. Photographic records were made with a VR-6 polygraph (Electronics for Medicine) at a paper speed of 100 mm/s. Traces were recorded under control and VT conditions, and after adenosine injection (immediately after its injection and then every 5 min for the first 15 min, and then every 30 min for the next hour or more). Traces were continuously followed on the polygraph screen. Systolic blood pressure was measured at the same time using a U-shaped mercury manometer attached to the right femoral artery (the variation in blood pressure was of more interest than the absolute values).
The recovery of SR within 15 min of the adenosine injection was considered an early effect. If the same occurred between 30 min and 60 min it was considered a delayed effect. Recovery was considered fleeting if it lasted only a few seconds, and transitory when it lasted a few minutes.
At the end of each experiment, the weight of the heart was recorded to determine the approximate received dose of adenosine in mg/g myocardium. The volumes of liquid administered to each animal and released in the urine were recorded.
Statistical Analysis
The results were assigned to the corresponding sections of a binary distribution table. Contrasts were made using the χ² test (95% confidence level). To determine the degree of association between variables, contingency coefficient C and the F2 indicator were calculated. All calculations were performed using either GB-STAT V 6.5 software for Windows or Wynks software (TexaSoft).
RESULTS
Control Group
Control animals showed neither fleeting nor transitory recovery of SR after the onset of VT. Thirty nine developed early VF while in 33 animals this developed 15 to 45 minutes after VT commencement, which accelerated progressively.
Anti-Arrhythmic Effect of Adenosine
In the preliminary study in 16 dogs with induced myocardial damage and VT produced by aconitine, SR was restored in 6 animals (37.5%). This was early but fleeting in 3, and delayed in the other 3 (data not shown in Tables).
Tables 1 and 2 show the results for experiments in which adenosine was administered independent of the origin of VT. The behavior of the course of VT in the animals treated with adenosine was significantly different to that seen in the control animals (no adenosine administered) (Table 1). Sinus rhythm was restored in 65% of those that received the agent, whereas none of the controls recovered SR. The heart rate in the SR restored by adenosine was equal to or less than that seen in control traces. In 35% of the adenosine-treated animals, no anti-arrhythmic effect was seen; VT persisted or even accelerated to VF.
Table 1 shows the contingency table for the 2 nominal variables: the horizontal referring to the administration (or not) of adenosine, and the vertical to its positive or negative effect in terms of SR recovery. The cells show the number of observations made. The statistical results reflected are those of the χ² test, the Yates correction, significance (95%) and the contingency coefficients Φ² and C. The effect of adenosine on the recovery of SR is doubtless, although a possible random effect cannot be ruled out, as shown by the association that approaches 1 (scale 0-1).
Table 2 shows that the 12 mg dose of adenosine led to a significantly more consistent delayed recovery of SR than did the 6 mg dose. It also demonstrates the cause-effect relationship of both doses (6 and 12 mg) with respect to early and delayed conversion. The results of the statistical tests demonstrate the more effective action of the high doses of adenosine on delayed reestablishment of SR. The results of the χ² test, the Fishers exact test, the Yates correction, significance (95%) and the contingency coefficients χ² and C are shown. The association between the higher dose and delayed conversion to SR is noticeable; the low dose restored SR early in 38% of animals, whereas the higher dose brought about delayed conversion in 52%. No significant difference was seen between the 2 doses in terms of early conversion to SR. Similar results were obtained when repeat doses were administered.
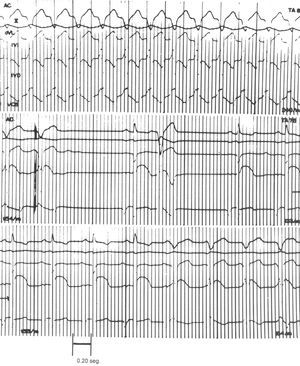
Figure 1 shows an example of the anti-arrhythmic effect of 6 mg of adenosine in an animal with induced myocardial damage and VT (240 beats/min) produced by aconitine with retrograde ventriculoatrial conduction (upper section). After receiving the adenosine bolus, VT was reduced to 154 beats/min. After a compensatory pause, a sinus complex and left ventricular extrasystole developed. Fleeting SR was recovered at 133 beats/min, lower than that seen for control SR (187 beats/min; middle section). In the lower section, at the right, the return to left VT (154 beats/min) can be seen. This and all other traces were recorded at a paper speed of 100 mm/s.
Figure 1.Anti-arrhythmic effect of the 6 mg dose of adenosine sulfate in ventricular tachycardia induced by aconitine in the damaged dog heart. Paper speed: 100 mm/s. RIC indicates right intraventricular complex; LIC, left intraventricular complex; AT, atrial tachycardia; SVC, superior vena cava.
In the 21 experiments performed with group B animals, in which two 6 mg doses of adenosine were injected, the first dose reestablished early, fleeting SR in 5 animals (24%). Delayed SR was reestablished in the same number. With the second dose, early, fleeting SR was reestablished in 5 animals (24%), and delayed SR was obtained in 2 (10%). The amount of adenosine administered was calculated as being between 0.04 mg/g and 0.06 mg/g of myocardium.
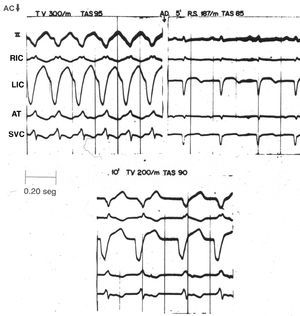
Of the 12 group C animals (which received a single 12 mg dose of adenosine), early, fleeting SR was restored in 8 (67%), and delayed, transitory SR obtained in 5 (41%). The amount of adenosine administered was calculated as between 0.05 mg/g and 0.16 mg/g of myocardium. Figure 2 shows an example of the anti-arrhythmic effect of the 12 mg dose of adenosine. The upper left section shows VT (300 beats/min) of homolateral origin induced by aconitine in a phenol-damaged heart. Five minutes after injection, SR was recovered (187 beats/min). Five minutes later (lower section), however, left VT reappeared (200 beats/min--lower than the first episode). The trace was made at a paper speed of 100 mm/s.
Figure 2. Anti-arrhythmic effect of the 12 mg dose of adenosine sulfate in ventricular tachycardia induced by aconitine in the damaged dog heart. Paper speed: 100 mm/s. SR indicates sinus rhythm; VT, ventricular tachycardia; RIC, right intraventricular complex; LIC, left intraventricular complex; SVC, superior vena cava.
In the 18 dogs with VT induced by myocardial damage, the first 12 mg dose of adenosine had an effect similar to that of the 6 mg dose; SR was restored early in 66.67% of animals. However, delayed SR was restored in a larger number of animals by the 12 mg dose (66.67%) than by the 6 mg dose (50% of animals).
With respect to the four animals with spontaneous VT, the first 12 mg dose led to early, fleeting reestablishment of SR in 1 (25%), and delayed reestablishment in the remaining 3 (75%). With the second dose, early, fleeting SR was restored in 2 animals (50%) and delayed SR was restored in 3 (75%). The amount of adenosine administered was calculated as between 0.01 mg/g and 0.12 mg/g of myocardium.
Pro-Arrhythmic Effects
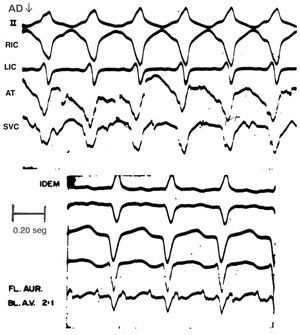
Supraventricular arrhythmias were seen in 5 of the animals that received two 12 mg doses of adenosine for VT induced by aconitine in a lesioned heart. In 1 animal, in which the drug had a delayed effect, low level atrial tachycardia developed (207 beats/min). In another, which had right VT, atrial flutter (461 beats/min) occurred immediately after the second 12 mg injection (Figure 3). This eventually provoked AF. The trace was recorded at a paper speed of 100 mm/s. Extrasystoles and Wolff-type tachycardia were also seen, as reported by other authors.20 One animal that weighed 14 kg, and which had a VT rate of 316 beats/min, suffered second degree ventriculoatrial block (type 2:1) after the second 12 mg injection. Sixty minutes after th is second injection, when the VT was 240 beats/min, second degree ventriculoatrial block occurred again with progressively longer Luciani-Wenckenbah-type periods. These unexpected findings were sporadic.
Figure 3. Atrial flutter (461 beats/min) and 2:1 atrioventricular block in traces taken immediately after the second 12 mg dose of adenosine was administered. The animal in question weighed 14 kg, its myocardium had been experimentally damaged, and VT had been induced by aconitine. Paper speed: 100 mm/s. BL.AV indicates BL atrioventricular block; ATFL atrial flutter.
DISCUSSION
Anti-Arrhythmic Effects of Adenosine
Aconitine is known to induce ventricular arrhythmias via the discharge of triggered potentials, against which adenosine is rightly considered an effective agent.21 The anti-arrhythmic action of adenosine, clearly seen in 68 (80%) of the 85 animals treated with this agent, may be associated with an automatism-depressing effect that leads to the start-up of the reentry mechanism. However, some authors22 believe that adenosine overcomes VT induced by triggered potentials via its electrophysiological effects on the ventricular myocardium, which are related to the inhibition of the cAMP/adenyl cyclase system. This anti-adrenergic action of the drug appears to be the best explanation for its ability to eliminate catecholamine-induced VT in human hearts.23 The fact that adenosine is unable to overcome VT due to reentry or ectopic automatism might be explained by its lack of any direct effect on the ventricular myocardium.24 However, in the present work, the fact that SR was restored early (both with the 6 mg and 12 mg dose) in 67% of the tachycardias due only to myocardial damage suggests that it is of benefit in ventricular arrhythmias probably due to reentries at the periphery of such damaged areas. Other authors, such as Dr. Josep Brugada of Maastricht, have reported the existence of such micro-reentries.
As an anti-arrhythmic agent, adenosine has many unusual properties. It is an intermediate metabolite with powerful negative chronotropic and dromotropic effects on the sinoatrial and atrioventricular nodes. It is also quick-acting and short-lived. These effects, as well as its anti-adrenergic action, form the basis of its anti-arrhythmic properties.24,25 Recently, the activating action of this agent on ATP-dependent potassium channels (K-ATP channels) has also been recognized. Ischemia/reperfusion studies on the dog heart have shown adenosine to have a protective effect related to the opening of these channels. This action is dependent on the activation of type A1 adenosine receptors and is antagonized by blockers of these such as glibenclamide, 8-cyclopentyl-1,3-dipropylxantine, and hydroxydecanoate.24,25 These studies have renewed interest in the ionic processes behind the electrophysiological actions of adenosine in the heart.26 Such actions are important in the analysis of the anti- and pro-arrhythmic properties of adenosine.
Although the action of adenosine on cardiac tissues is short-lived (the mean half life of the agent is between 1 and 6 s),27 its phosphorylation to AMP takes 2 h or more. Therefore, the early, fleeting anti-arrhythmic effect of adenosine, observed just after its injection, is probably due to the drug itself. The reduction in systolic blood pressure is probably similar, which in general is immediate and transitory.28 However, the delayed anti-arrhythmic effect (30 to 60 minutes after administration of the drug) could be due to some derivative or catabolite with a more prolonged action than the original compound. Future work should include investigations into whether the catabolic products of adenosine have the same kind of anti-arrhythmic action against experimental VT. Some authors believe one such product, inosine, to be inactive.19
The χ2 test was believed appropriate for evaluating the effect of adenosine in the restoration of SR.29,30 The difference in SR reestablishment between animals that received adenosine and those that did not was very significant (Table 1). The aim of the F2 indicator (Pearson, 1900)31,32 and the C contingency coefficient (Pearson, 1904) is to determine the degree of the association between variables. The calculation of these values is a basic step in attributing cause-effect relationships.
The anti-arrhythmic action of adenosine in VT due only to myocardial damage suggests the same action may be possible with certain reentries.
Pro-Arrhythmic and Negative Dromotropic Action of Adenosine
The atrial tachycardias sometimes observed were probably due to sympathetic reflexes caused by the adenosine.16 Patch-clamp techniques have shown that adenosine activates K+ channels in a fashion similar to acetylcholine.33 Such increases in potassium conductance shorten the duration of the action potentials in atrial myocardial cells,34 cause their hyperpolarization, and induce atrial automatism. The shortening of the refractory period of these cells explains the appearance of atrial flutter and later fibrillation. These effects of adenosine on K+ channels also occur in the cells of the sinoatrial and atrioventricular nodes. Atrioventricular conduction is greatly reduced because adenosine reduces the amplitude and duration of the action potentials.35 This is accentuated in the cells of the central region of the atrioventricular node36 and are thought to be due to the direct action of adenosine on K+ conductance. The indirect action of adenosine on the calcium inflow current may also be important in certain situations. Anterograde (atrioventricular)37 and sometimes retrograde (ventriculoatrial)38 blocks are therefore seen. The action of adenosine on the tissues situated under the node is more variable.
Limitations of the Study
The number of high dose (12 mg) experiments performed was small. A larger number might confirm the observations made and support the hypothesis that the derivative of adenosine has greater anti-arrhythmic action than adenosine itself, as suggested by the greater number of delayed restorations of SR with this dose (Table 2) and their longer duration.
CONCLUSIONS
In conclusion, adenosine exerts an anti-arrhythmic effect on VT induced by triggered potentials (due to aconitine) and may do so on those maintained by micro-reentries at the periphery of damaged areas of the myocardium (when VT is caused exclusively by these lesions). The latter is an inference based on the results seen when no aconitine was administered to induce triggered potentials. This requires direct confirmation.
Adenosine has a biphasic anti-arrhythmic effect: one early and short-lasting, the other delayed and sustained. Since adenosine has a very short half-life, it is proposed that the first is due to the action of the agent itself, and the second to a derivative or catabolite of adenosine.
Correspondence: Dr. A. de Micheli.
Juan Badiano #1. Sección XVI. Tlalpan. Distrito Federal. México.
E-mail: archivos@cardiologia.org.mx