Keywords
INTRODUCTION
Atrial fibrillation and atrial flutter are 2 common arrhythmias. Patients who primarily present atrial fibrillation generally also experience atrial flutter over the course of their lives, and vice versa.1,2
Since these arrhythmias differ in their underlying physiologic mechanisms and the currently available therapeutic options for their treatment, it is essential to clearly differentiate between them.3,4
Differential diagnosis between atrial fibrillation and atrial flutter sometimes presents problems that are difficult to resolve, even with the use of endocardial electrograms. This is especially true when recordings are obtained with a single catheter electrode in the right atrium, as occurs, for example, with devices used for treatment of supraventricular tachyarrhythmia, since some episodes of atrial fibrillation can present with an organized electrical pattern in that chamber.
The aim of this study was to identify an electrophysiologic parameter that is easily identified with a single catheter electrode in the right atrium and that allows discrimination between atrial flutter and organized atrial fibrillation.
METHODS
In patients requiring an electrophysiologic study for ablation of atrial fibrillation, typical atrial flutter, or atypical atrial flutter (focal tachycardias were excluded), a 24-pole mapping catheter (Orbiter®, Bard Electrophysiology) was introduced over the coronary sinus and the lateral tricuspid annulus. The catheter was used to obtain simultaneous bipolar recordings from the right atrium (lateral tricuspid annulus and cavotricuspid isthmus) and the left atrium.
Patients were selected from among individuals referred to our hospital for ablation of atrial fibrillation (n=32), typical atrial flutter (n=81), and atypical atrial flutter (n=15) who at the time of the electrophysiologic study presented the arrhythmia to be treated or who developed the arrhythmia spontaneously or with programmed stimulation during the procedure, and who met the inclusion criteria. The cases of atrial fibrillation had to display an organized electrical pattern (defined as a fixed and reproducible sequence) in the right atrial recordings over a period of at least 30 seconds and a disorganized pattern (fragmented signals, defined as atrial recordings with a duration of at least 150% of the baseline atrial electrogram or lasting more than 100 ms) in the coronary sinus (Figure 1). Typical atrial flutter was defined as that which was dependent on the cavotricuspid isthmus, while atypical atrial flutter was defined as any macroreentrant arrhythmia presenting with a completely organized electrical pattern in the 12 electrodes of the recording and that was not dependent upon the cavotricuspid isthmus, using programmed stimulation.
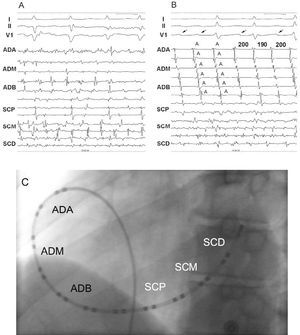
Figure 1. A and B: show surface and endocardial recordings in 2 patients with atrial fibrillation. In (A), irregular and fragmented signals are observed in the channels corresponding to both atria, while in (B), irregular, fragmented signals are seen in the channels corresponding to the left atrium alongside organized signals in the channels corresponding to the right atrium. An organized pattern is seen in the V1 lead of the electrocardiogram (arrow). C) Left anterior oblique projection showing the position of the 24-pole mapping catheter in the coronary sinus and right atrium. I, II, V1 indicate electrocardiography leads; A, atrial electrogram; HRA, high right atrium; MRA, middle right atrium; LRA, low right atrium; PCS, proximal coronary sinus; MCS, middle coronary sinus; DCS, distal coronary sinus.
Cycle length was measured along with the variation in each atrial cycle in the electrograms obtained in the high right atrium, in the vicinity of the right atrial appendage (where the atrial wire electrode of pacemakers for treatment of bradyarrhythmias or tachyarrhythmias is usually situated), in 3 consecutive bipolar channels over a period of 15 seconds. Recordings were obtained at a chart speed of 400 mm/s in an effort to ensure that they were as accurate as possible.
The patients included in the study had not had a previous electrophysiologic study and were not receiving antiarrhythmia treatment at the time of the study.
Statistical Analysis
Data are expressed as means (SD). Quantitative variables were compared by analysis of variance and the Bonferroni correction was used to establish the differences between the variables.
Receiver operating characteristic curves were used to determine the cut point for quantitative variables. The area below the curve was determined along with the 95% confidence interval (CI). The criterion validity of the tests was calculated for the maximum sensitivity and specificity in the diagnosis of atrial flutter: sensitivity, specificity, positive and negative likelihood ratio, and positive and negative predictive values. A P-value less than .05 was considered statistically significant. Statistical analysis was performed using SPSS (version 13.0) for Windows.
RESULTS
The study included a total of 45 patients: 15 patients with atypical atrial flutter, 15 with typical atrial flutter, and 15 with atrial fibrillation. The mean age of the patients was 58 (11) years, 28 patients (62%) were men, 19 (42%) had arterial hypertension, 6 (13%) had diabetes mellitus, and 12 (27%) had structural heart disease (hypertensive in 7 patients [16%], ischemic in 4 [9%], and valvular in 1 [2%]). The mean size of the left atrium was 43 (6) mm. No significant differences were observed in the baseline characteristics of the 3 groups, with the exception of age, which was significantly lower in the group of patients with atrial fibrillation (Table).
The patients with typical and atypical atrial flutter already had the arrhythmia at the time of the electrophysiologic study, and of the 15 patients with atrial fibrillation, 10 presented with the arrhythmia and 5 developed sustained arrhythmia (more than 10 minutes) induced by programmed atrial stimulation.
In the patients with atrial fibrillation, the activation front in the right atrium was in a craniocaudal direction in all cases, and an F wave was always discernible in the V1 lead of the surface electrocardiogram that was stably associated with endocardial activation (Figure 1).
Cycle Length
The cycle length measured in the bipolar electrograms of the right atrium was 232 (21) ms in the patients with atypical atrial flutter (range, 224 [22] to 240 [21] ms), 234 (24) ms in the group with typical atrial flutter (range, 227 [24] to 240 [25] ms), and 183 (16) ms in the group with atrial fibrillation (range, 172 [15] to 194 [17] ms) (P=NS between the groups with typical and atypical atrial flutter and P<.05 between the 2 groups with atrial flutter and the group with atrial fibrillation). A cycle length ≥203 ms allowed discrimination between atrial flutter and atrial fibrillation with a sensitivity of 97% (95% CI, 90.2%-100%) and a specificity of 87% (95% CI, 69.5%-100%) (P<.001) (Figures 2A and 3A). The positive likelihood ratio for this value was 7.25 (95% CI, 1.99-26.39) and the negative likelihood ratio, 0.04 (95% CI, 0.01-0.27). The positive predictive value with this cut point was 94% (95% CI, 84.9%-100%) and the negative predictive value, 93% (95% CI, 79.4%-100%).
Figure 2. A) Cut point for discrimination between both groups of patients with atrial flutter and patients with atrial fibrillation in terms of cycle length. B) Cycle length variation in the 3 groups. CL indicates cycle length.
Cycle Length Variation
The cycle length variation was 16 (7) ms in the atypical atrial flutter group, 13 (4) ms in the typical atrial flutter group, and 22 (7) ms in the atrial fibrillation group (P<.05 between typical atrial flutter and atrial fibrillation; P=NS between the other groups) (Figure 2B). A cycle length variation ≤18 ms allowed discrimination between atrial flutter and atrial fibrillation with a sensitivity of 70% (95% CI, 53.6%-86.4%) and a specificity of 80% (95% CI, 59.8%-100%) (Figure 3B). The positive likelihood ratio for this value was 3.5 (95% CI, 1.24-9.89) and the negative likelihood ratio, 0.38 (95% CI, 0.21-0.67). The positive predictive value with this cut point was 88% (95% CI, 74.3%-100%) and the negative predictive value, 57% (95% CI, 36%-78.3%).
Figure 3. Receiver operating characteristic curves for cycle length (A) and cycle length variation (B). AUC indicates area under the curve; CI, confidence interval.
Cycle Length and Variation
Using a combination of both criteria to discriminate between atrial flutter and atrial fibrillation (cycle length ≥203 ms and cycle length variation ¾18 ms) yielded a sensitivity of 99%, a specificity of 69%, a positive predictive value of 26%, and a negative predictive value of 99%.
DISCUSSION
Principal Findings
Two main observations were made in our study. Firstly, that there are significant differences in right atrial cycle length and cycle length variation between atrial flutter and atrial fibrillation that presents with an organized pattern. Cycle length was greater and cycle length variation lower in atrial flutter than in atrial fibrillation, with no significant differences between the 2 types of atrial flutter. Secondly, cycle length was a better parameter than cycle length variation to differentiate between atrial flutter and this subset of atrial fibrillation. A cycle length ≥203 ms allowed discrimination between atrial flutter and atrial fibrillation with a good sensitivity and specificity; atrial flutter was found to be 7.25 times more likely when the cycle length is above this cut point and it is 25 times more likely that this diagnosis is ruled out when a value for cycle length below the cut point is obtained. A slight increase in the sensitivity of the test was observed when the 2 parameters were combined, but this was associated with a significant reduction in specificity. Consequently, the use of a combination of the 2 variables did not improve the diagnostic yield.
Minimum cycle length was not considered in the analysis, since this parameter could contain artifacts that would generate excessively short intervals and lead to overestimation of the diagnosis of atrial fibrillation.
Organized Atrial Fibrillation in the Right Atrium
Currently, there is no simple definition of atrial fibrillation that is applicable to both electrocardiograms and electrophysiologic recordings. It has been reported that atrial fibrillation and atrial flutter represent 2 arrhythmias that are interrelated and that, via different mechanisms, each participates in the genesis of the other.5 However, both arrhythmias can coexist in the same patients, making differential diagnosis difficult, especially when it involves atypical atrial flutter or organized atrial fibrillation. This type of atrial fibrillation is characterized by a disorganized pattern of endocardial activation in the left atrium and an organized pattern in the right atrium, as has been described by some authors6-9 and as we have illustrated in this study. In a case series involving 16 patients with atrial fibrillation in whom endocardial mapping was performed at different sites in the right atrium and coronary sinus over a period of 50 minutes, Roithinger et al9 observed that in up to 72% of the time period there was organized activation in the trabecular region of the right atrium, compared with only 19% of the time in the smooth wall of that chamber and 51% of the time in the coronary sinus. However, this has not been studied systematically in the population of patients with atrial fibrillation, and consequently, its prevalence, clinical significance, and therapeutic implications are unknown, and no clear explanation of the underlying mechanism is available. One possible hypothesis to explain this pattern of electrical activation in some patients is that the primary circuit responsible for sustaining atrial fibrillation is located in the left atrium, while the right atrium is passively activated. In this context, interatrial conduction has been studied and it has been demonstrated that both atria communicate with each other via preferential routes of conduction located around the ostium of the coronary sinus, the fossa ovalis, and the anterosuperior region of the interatrial septum.10 It is likely that, similar to events associated with the genesis of atrial flutter, this pattern of electrical activation in the right atrium observed during atrial fibrillation occurs as a result of a functional block via the crista terminalis. Thus, fibrillatory conduction arising from the left atrium, modulated in some way through Bachmann's bundle and the other preferential fibers, would be organized and display an organized activation pattern in a craniocaudal direction in the lateral wall and in a caudocranial direction in the septal wall of the right atrium, or vice versa, imitating the activation pattern of atrial flutter dependent upon the isthmus.11 This modulation of conduction between the left and right atria has been studied to some extent by O'Donnell et al.12 Those authors analyzed the refractory periods and conduction times of Bachmann's bundle and the ostium of the coronary sinus in patients referred for ablation of atrial fibrillation. They observed that both the refractory periods and the delayed conduction through those structures during decremental pacing from the left atrium were significantly greater in patients with atrial fibrillation than in a control group, indicating that those structures display an electrophysiologic behavior that varies in each patient.12
Clinical Usefulness
Rapid detection of atrial tachyarrhythmias and reliable discrimination between atrial flutter and atrial fibrillation have important clinical implications for the use and programming of pacemakers and defibrillators that can deliver atrial therapies. In these patients, atrial antitachycardia pacing is reported to be effective in 30% to 50% of the episodes, depending on the study.13-15 It is known that atrial arrhythmias (atrial tachycardia, atrial flutter, and atrial fibrillation) are interrelated, and in this sense, the low relative efficacy of pacing therapy may be due to an incorrect interpretation of the arrhythmia detected by the device, even more so if atrial detection is performed by a single catheter electrode implanted in the right atrium. This could be the cause of detection errors and ineffective therapy. However, from a clinical perspective, it is advisable that the device be programmed in such a way that significant underdetection of episodes of atrial flutter does not occur, even though some episodes of atrial fibrillation are treated by antitachycardia pacing. This easily applied algorithm is also of practical use during electrophysiologic studies of patients with tachycardias in those cases in which only a single catheter electrode is available in the right atrium, in order to be able to rapidly discriminate between atrial flutter and organized atrial fibrillation.
Previous Studies
Various methods have been used in an effort to differentiate between atrial flutter and atrial fibrillation. Some of those methods are easy to apply. Jung et al16 studied 28 patients and found that a mean cycle length of more than 315 ms discriminated those patients with normal sinus rhythm from those with atrial flutter or atrial fibrillation, and that an SD of more than 11.5 ms discriminated those with atrial fibrillation from those with atrial flutter. However, that study did not specify whether or not the episodes of atrial fibrillation presented an organized activation pattern in recordings from the right atrium. Other authors have employed complex methods that require the use of specific analyzers to discriminate between atrial flutter and atrial fibrillation, such as evaluation of the intersignal variability using a transform and calculation of the SD for different scales,17 Bayesian analyses with a series of elements such as regularity, rate, energy distribution of the obtained signals, etc,18 and time-domain analysis of the QRS complex-subtracted electrocardiogram.19 In general, all of these proposed algorithms display an adequate diagnostic yield to obtain a differential diagnosis between the 2 arrhythmias. However, their use is essentially limited to research applications because of the time required for their analysis and the need for specific technology.
Limitations
The main limitation of our study is that the patients with atrial fibrillation belong to a selected population of patients referred for ablation of atrial fibrillation. As such, the results might not be applicable to the general population of patients with atrial fibrillation.
CONCLUSIONS
Cycle length and cycle length variation in electrograms recorded from the right atrium are significantly different between atrial flutter and organized atrial fibrillation in the right atrium, with a longer cycle length and lower cycle length variation in atrial flutter. A cycle length >=203 ms allowed discrimination between atrial flutter and atrial fibrillation with good sensitivity and specificity. Cycle length variation did not improve the diagnostic yield in distinguishing between the 2 arrhythmias.
Correspondence: Dr. J. Villacastín.
Unidad de Arritmias. Instituto Cardiovascular. Hospital Clínico San Carlos. Prof. Martín Lagos, s/n. 28040 Madrid. España.
E-mail: jvillacastin@secardiologia.es
Received December 12, 2005.
Accepted for publication November 2, 2006.