Keywords
INTRODUCTION
Cardiovascular disease (CVD) is the main cause of death in western countries.1 The classic cardiovascular risk factors, such as diabetes, hypertension (HT), and smoking are not present in 10% to 20% of cardiovascular events; hence, there is a need to find new markers that will increase the precision of predicting cardiovascular risk (CVR).2
Chronic renal disease (CRD) is often associated with CVD and considerably increases the patient's risk status. Recent studies have shown that even mild renal impairment is related to this elevated risk,3 and this fact has led to the idea that markers of renal function may be true sentinel indicators of CVR.
The best marker of renal function is the glomerular filtration rate (GFR), but it is difficult to measure the GFR in daily practice. For this reason, the serum creatinine concentration or equations derived from this parameter have been used to calculate the estimated glomerular filtration (eGF). These estimations present limitations4 that make it difficult to detect renal disease in the initial stages.
Cystatin C (CC) is a low-molecular-weight (13 kDa), nonglycosylated protein from the family of cysteine protease inhibitors that closely approximates what could be considered an ideal marker of renal function.5 It is more sensitive than creatinine for detecting slight decreases in glomerular filtration and could be useful for early diagnosis of CRD and as a predictor of CVR.
Various publications have demonstrated the value of CC for predicting CVR in elderly persons.6,7 Few studies, however, have provided data on this marker in the younger population or, more importantly, in the overall population. This information is essential to improve the interpretation of CC values and enable their application in clinical practice.
Aims
To determine the prevalence of elevated CC levels and the association between this finding and cardiovascular risk factors and cardiovascular disease in the general population.
METHODS
Patients
A descriptive epidemiologic cross-sectional study was carried out in a sample of persons older than 49 years taken from the general population (n=76 660), obtained by simple random sampling from the public healthcare database for residents of the city of Oviedo (Spain).
The patients analyzed had participated in a previous study designed to determine the prevalence of peripheral arterial disease in the general population. Assuming a prevalence of 12%, an alpha error of .05, and a desired precision of 0.03, 415 individuals were selected. All patients gave informed consent at the start of the study. Patients with end-stage disease and immobile patients were excluded. Patients who died, moved to another area, or refused to participate were considered lost and were not replaced. From the total number of patients initially selected, serum samples from 359 individuals were frozen and stored at -70°C.
Because there are no previous studies investigating elevated CC levels in the general population, we used the prevalence of CRD as a reference to calculate the minimum sample size, based on the idea that at least patients with this disease would have elevated CC, which is a very sensitive parameter for detecting CRD. In our setting, the prevalence of CRD is 11%,8 and with this figure we estimated a minimum sample size of 306 patients (alpha error, .05, and desirable precision, 0.03). The study was approved by the ethics committee for clinical research of our hospital.
Measurements
A protocol was designed to collect demographic data (age, sex), clinical data (weight, height, body mass index, systolic blood pressure, diastolic blood pressure), cardiovascular risk factors (smoking, dyslipidemia, HT, diabetes, obesity) and cardiovascular conditions (ischemic heart disease, heart failure, cerebrovascular disease, peripheral arterial disease). Information on the treatment used for HT, hyperlipidemia, and diabetes, as well as anticoagulant/antiplatelet therapy was recorded.
All patients underwent a comprehensive laboratory workup, including a complete blood count, and analysis of fibrinogen, glucose, creatinine, total cholesterol, high-density lipoproteins, triglycerides, uric acid, lipoprotein (a), hemoglobin A1c, homocysteine, C-reactive protein, and CC. In a single urine sample, the albumin:creatinine ratio was calculated. Samples were processed on the same day as extraction, except in the case of CC and homocysteine, which were determined in aliquots that had been stored at -70°C. Samples were processed according to the recommendations of the manufacturer of the analytical technique used. CC was determined with an immunonephelometric method (N Latex cystatin C, Dade Behring), which had a between-run coefficient of variation (CV) of 2% to 2.8% and a within-run CV of 2.3% to 3.1%; the normal range for adults is 0.51 to 0.95 mg/L.9 Creatinine was measured with a kinetic method (Roche-modular), CRP with turbidimetry (Roche-modular), and homocysteine with a chemiluminescent technique (Advia-Centaur).
Definition of the Variables
Cystatin C was considered elevated when it exceeded the upper limit of normal recommended for the technique (>0.95 mg/L).9 To estimate renal function, that is, the eGF, the abbreviated Modification of Diet in Renal Disease (MDRD) equation was used, in keeping with the criteria of the 2002 Kidney Disease Outcome Quality Initiative (KDOQI) guidelines.1 HT was established based on a systolic blood pressure ≥140 mm Hg and/or a diastolic blood pressure ≥90 mm Hg in at least 2 out of 3 separate visits, or on the fact that a patient was receiving dietary or pharmacologic antihypertensive therapy. Patients were considered diabetic when the baseline glucose concentration was >126 mg/dL on 2 occasions, the oral glucose tolerance test yielded results of >200 mg/dL at 2 hours, or when patients were already receiving antidiabetic treatment with insulin or oral antidiabetic agents. A smoker was defined as a person who had smoked in the previous month, and an ex-smoker was a person who had smoked at one time, but had not smoked in the previous year. All patients receiving lipid-lowering drugs were considered to have hypercholesterolemia, as well as those with total cholesterol values >240 mg/dL in 2 analyses more than 3 weeks apart or hypertriglyceridemia, based on values >200 mg/ dL in 2 separate analyses. Microalbuminuria was defined as an albumin:creatinine ratio of 30 to 300 mg/g. Obesity was defined as a body mass index (weight in kilograms divided by height in meters, squared) ≥30.
Cardiovascular comorbidity included ischemic heart disease (angina and acute myocardial infarction), heart failure, cerebrovascular disease, and peripheral arterial disease, when documented during hospitalization or after a specialized study.
Statistics
The prevalence of individuals with elevated CC in the general population was calculated with the 95% confidence interval (CI). To determine associations between CC and the various factors studied, the t test for independent samples was used. Analysis of variance was applied to compare the means of quantitative variables. The χ2 test (or Fisher exact test) was used for qualitative variables and to study differences between the quartiles of CC after stratification. Logistic regression analysis was performed to determine which factors were independently associated with CC elevations. Significant variables in the univariate analysis were included in the model.
Results are expressed as the mean (SD) or as percentage. Statistical significance was established as P<.05 in all calculations. The statistical treatment was carried out using SPSS (version 12.0).
RESULTS
Cystatin C determinations were performed in 359 patients (women, 63.5%; mean age, 64 [9.83] years; and range, 50-98). The study group did not differ significantly from the randomly selected initial group in demographic characteristics or vascular risk factors.
The demographic, clinical, and laboratory characteristics of the study group are presented in Table 1.
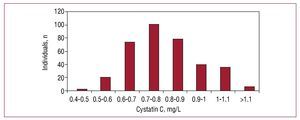
The prevalence of elevated CC values in the population was 17.3% (95% CI, 13.4%-21.2%), with a mean of 0.8 (10.21) mg/L (0.8 [0.17] mg/L in women and 0.84 [0.25] in men, with no significant differences). The distribution of CC values in the population studied are shown in Figure 1.
Figure 1. Distribution of cystatin C values in our population.
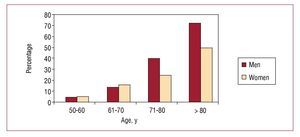
The prevalence of elevated CC increased with advancing age. Elevated values were found in only 5.5% of the youngest group (50-60 years), followed by 15% of those aged 61 to 70, 32% of those 71 to 80, and 61.3% of those older than 80 years. The prevalence was very similar between sexes up to age 70, after which time elevated CC levels became more prevalent in men than women (Figure 2).
Figure 2. Prevalence of elevated cystatin C according to age and sex.
At least one of the classic cardiovascular risk factors was present in all middle-aged patients with elevated CC: HT (55%), diabetes (33%), and smoking (44%).
An association was found between CC elevation and the presence of HT (P=.004), ischemic heart disease (P=.013), and heart failure (P=.007), as well as several parameters related to CVR. CC elevation was associated with more CVR factors than the eGF decrease, calculated with the MDRD equation (Table 2).
Following stratification of CC concentrations into quartiles, a significant relationship was found for microalbuminuria and C-reactive protein and fibrinogen increases as well as high-density lipoprotein cholesterol (HDL-C) and eGF decreases, as CC values progressively increased (Table 3).
In the logistic regression analysis, the main factors independently related with elevated CC concentrations were diabetes (odds ratio [OR] =5.37), male sex (OR=4.91), and decreased eGF (OR=0.83) (Table 4).
DISCUSSION
We present the first study performed in Spain that determines the prevalence of elevated CC levels and their relationship with CVR factors in the general population. Up to now, this issue has been examined mainly in specific populations, such as elderly persons11 and patients with renal disease12 or hypertension.13 The first articles dealing with the general population have appeared only recently. In the NHANES III (Third National Health and Nutrition Examination Survey). Köttgen et al14 reported a 9.6% prevalence of elevated CC levels in the general population using the same measurement method as in the present study, but applying the 99th percentile (1.12 mg/L) as the upper limit of normal. Later, Parikh et al,15 in the Framingham Offspring study, which contained a population with characteristics similar to ours, reported a 22% prevalence of elevated CC levels, using the 95th percentile (1.07 mg/dL) as the upper normal limit.
These differences in prevalence may be the result of differences in the populations studied, in the definitions of normal limits, or in the calibration of the analytical methods used, making comparisons between the studies difficult. Our data are similar to the findings of Parikh et al and are consistent with the estimated prevalence of CRD in our setting, which ranges from 7.5% to 17.8%, depending on the method used to estimate renal function.8,16
Other authors17 have described CC elevations with advancing age and have attributed them to progressive deterioration of renal function, although the lack of direct measures of glomerular filtration in the present study and in others is an obstacle to precise determination of this relationship. It is known that the prevalence of CRD increases considerably after the age of 70, and it is precisely at this age when CC concentrations began to show substantial increases in this study and in others.17
Our results also coincide in that elderly men present higher CC values than elderly women; nevertheless, in younger patients, we found similar values in both sexes.
Patients with classic CVR factors presented high rates of elevated CC levels, particularly those with microalbuminuria (35%), which is a well-recognized independent factor for CVR and mortality. In contrast to other studies,18 higher CC levels were not found in our population of smokers, and decreased eGF was associated with the lowest use of tobacco. This association disappeared after adjusting for CVD, however, and it can be explained by the fact that patients with heart disease tend to smoke less and usually have a lower eGF.
Patients with established CVD presented the highest prevalence of elevated CC levels, and, as is well recognized, these patients are at a high risk of experiencing new cardiovascular events. This association has been demonstrated in numerous studies and is attributed to the relationship between CC and CRD, but recent publications have shown that CC elevations increase the prevalence of CVD even in patients without CRD, which suggests that CC would be a better biomarker of CVR than eGF.15,19 One recent study performed in our country in patients with acute coronary syndrome showed that those with the highest CC values presented a poorer cardiovascular prognosis, and this was true even in the group with normal eGF results; these findings may have implications for risk stratification in this group of patients.20
Shlipak et al6 have described the relationship between CC and proinflammatory parameters, such as C-reactive protein and fibrinogen in the elderly population. Our findings support the idea that this close association is maintained in the younger general population, and that it is gradual and progresses with the CC rise.
In the Multi-Ethnic Study of Atherosclerosis (MESA), Keller et al21 reaffirm these data and show that CC is associated with an extensive battery of inflammatory and procoagulant markers in all aspects of renal function, whereas eGF only shows a relationship when its decreases reach <60 mL/min.
One explanation for this situation could be that the GFR is linearly associated with inflammation, and because CC is a more sensitive marker than the GFR, it would show a closer association with these molecules. Another possible explanation is that CC is associated with inflammation regardless of renal function, as some authors have suggested,18,21 although the majority, including those reporting two meta-analyses,5,22 agree that CC is a very sensitive marker of small decreases in renal function.
In our population, patients with elevated CC levels showed more associations with CVR factors than patients with eGF decreases, and it was seen that C-reactive protein and fibrinogen increases and HDLc decreases were associated with elevated CC, but not with decreased eGF.
The factors that were independently associated with elevated CC levels in the general population were diabetes, male sex, and decreased eGF. In the general population of the United States, Köttgen et al14 found a similar association between CC and diabetes in the group of patients 50 to 60 years old, the age at which the incidence of CRD begins to increase. This metabolic disease is a classic example of an early, silent kidney disease that confers an elevated CVR. In patients older than 60, C-reactive protein and advanced age, together with other factors that accompany ageing, were the variables most closely related to elevated CC levels.
In hypertensive patients in Spain, Rodilla et al13 also found an association between CC and C-reactive protein, but the factor most closely related to CC was eGF. The reason why CC elevations rather than eGF decreases are first associated with diabetes can be attributed to the fact that CC increases occur with only mild GFR decreases (between 70 and 80 mL/min),23 whereas the eGF is only considered abnormally low at values <60 mL/min. Thus, diabetic patients could present a mild renal alteration detected by CC analysis while the eGF has not yet reached abnormally low levels.
Recent studies have shown that CC is associated with metabolic syndrome24 and can predict the development of HT in the general population without previous renal or cardiovascular disease,25 prediabetes,26 or diabetic nephropathy.27 Thus, this test is superior to microalbuminuria as a predictor of HT because of its more precise estimation of changes in glomerular filtration.
Based on this background, it can be suggested that CC measurement may identify persons in the general population with mild vascular injury, a condition that often precedes diseases such as diabetes and HT, and whose identification would be very useful for establishing appropriate treatment and, particularly, prevention measures.
It seems that the patients who would most benefit from this test would be older persons, women, and diabetic patients with normal renal function, in whom creatinine analysis, GFR formulas, or microalbuminuria do not always reveal alterations. Only CC elevation could alert the physician to the increased vascular risk in these patients.
Our study has some limitations. First, because of its descriptive, epidemiologic, cross-sectional design, we can only propose a hypothesis about the potential usefulness and advantages of CC determination. We cannot demonstrate these advantages because the study was designed to investigate the prevalence of elevated CC levels, but not associations with the various CVR factors. Prospective studies designed to determine the true cause of the association between CC and CVD would be needed.
The study focused on the general population older than 49 years because it is the group at the highest risk of renal and vascular disease, in which the greatest diagnostic yield was expected. Thus, the findings are only applicable to this population group. The lack of standardized creatinine and CC measurement methods also makes extrapolation of the results difficult. The fact that only one measure of eGF and microalbuminuria was available may imply some bias in the classification of patients, but we believe that the large number of individuals included may mitigate these possible errors, so they would not have a substantial influence on the final results. CC concentration can be affected by several factors, such as thyroid disease and corticoid use,17 which were not excluded from our study; nonetheless, because of the low prevalence of these factors in the general population, we also believe that they would not significantly alter the final results.
CONCLUSIONS
In our setting, we found a high prevalence of individuals with elevated CC concentrations, which were related to classic cardiovascular risk factors such as diabetes, CRD and HT, and with emergent CVR markers such as C-reactive protein, homocysteine, and fibrinogen. If the association between CC and CVD is confirmed in other studies, this test could become a useful tool in screening for vascular diseases, facilitating early diagnosis and adequate treatment and leading to a considerable improvement in the management of these diseases and in reducing their morbidity and mortality.
Additional studies are needed in the general population to confirm these data and to provide more information on the possible advantages of CC determination versus other tests.
ACKNOWLEDGMENTS
We wish to express our sincere thanks to Dr Vicente García (Gerencia de Atención Primaria, Área Sanitaria V, Gijón) and Dr Pablo Herrero (Hospital Universitario Central de Asturias, Oviedo) for their assessment of the methods and the rigorous statistical treatment performed in this study.
ABBREVIATIONS
CC: cystatin C
CRD: chronic renal disease
eGF: estimated glomerular filtration
HT: hypertension
GFR: glomerular filtration rate
MDRD: Modification of Diet in Renal Disease
This study was funded in part by a grant from semFYC (Sociedad Española de Medicina Familiar y Comunitaria).
Correspondence: Dr. J. Cepeda Piorno.
Hospital Santos Reyes.
Avda. Ruperta Baraya, 6. 09400 Aranda de Duero. Burgos. España.
E-mail: j_cepeda_p@hotmail.com
Received March 28, 2009.
Accepted for publication November 30, 2009.