Coronary lithoplasty (CL) is a balloon-based technique used to treat calcified lesions. This study reports the initial experience of treatment of calcified lesions with CL in an unselected and high-risk population.
MethodsThis was a prospective, multicenter registry, which included all consecutive cases with calcified coronary lesions that underwent CL between August, 2018 and August, 2019. Exclusion criteria consisted of a target lesion located in a small vessel (< 2.5mm) and the presence of dissection prior to CL. Quantitative coronary angiography and intravascular ultrasound/optical coherence tomography analysis were completed by an independent central core laboratory.
ResultsThis registry included 57 patients (66 lesions). The population was elderly (72.6±9.4 years) with high proportions of patients with diabetes (56%), chronic kidney disease (35%), and multivessel disease (84%). All lesions were classified as type B/C. More than 75% of lesions were predilated with noncompliant/semicompliant balloons or cutting-balloon. Rotablator was used in 5 lesions (7.6%) prelithoplasty. On average, CL required 1.17 balloons delivering a mean of 60 pulses. Successful CL was achieved in 98%. In 13% of cases, lithoplasty balloon was broken during therapy. There were few procedural complications: 2 cases of significant dissections (none related to lithoplasty balloon rupture) were successfully treated with drug-eluting stent implantation. One patient experienced stent thrombosis 2 days after successfully undergoing target lesion revascularization.
ConclusionsThis is a real-world multicenter registry, which supports the feasibility, safety, and short-term efficacy of PCI for calcified coronary lesions using CL in an unselected and high-risk population with promising results.
Keywords
Calcified coronary lesions (CCL) are challenging to treat with percutaneous coronary intervention (PCI).1 Population aging and growing prevalences of diabetes mellitus and chronic kidney disease are associated with an increase in coronary calcification.2 The severity of endoluminal calcium may be underestimated by coronary angiography or fluoroscopy, so intravascular imaging techniques, such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT), are required to achieve a meticulous assessment of the severity and characterization of the lesion.3
Severe coronary calcium increases PCI complexity. It may impair the crossing of the lesion, affect suitable stent expansion and apposition, damage the drug-eluting polymer, increase the risk of stent thrombosis and restenosis, and adversely impact acute and long-term results after PCI.4 The optimal approach to the treatment of CCL requires knowledge of several factors: lesion characteristics, calcium distribution, intravascular imaging techniques, and the mechanism of each plaque-modification device.5
Available techniques for the treatment of CCL can be divided into 2 groups: nonballoon and balloon-based technologies.5,6 Plaque-modification techniques include nonballoon technologies such as rotational atherectomy (RA), orbital atherectomy, and excimer laser.6,7 Balloon-based technology may increase the lumen diameter by cracking the calcium circumferentially and allowing greater elasticity of the lesion.8
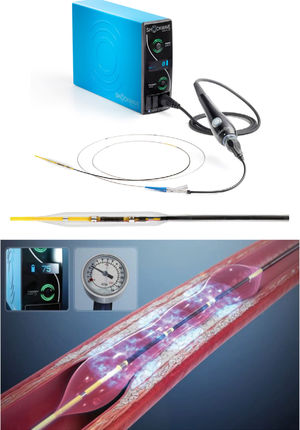
We recently described the successful initial experience with coronary lithoplasty (CL) for modification of CCL in a series of 3 cases.9 CL is a new technique: it involves the use of high-energy mechanical pulses, delivered by a semicompliant balloon, to crack coronary calcium.8 The lithoplasty balloon (LB) is inflated at a pressure of 4atm in the target calcified lesion and delivers pulsatile mechanical waves at a frequency of 1Hz.8,9 The mechanical energy is transmitted to the lesion when the LB contacts the intima layer, allowing the fracture of calcium in the superficial and deeper coronary wall layers.10 These shockwaves allow adequate plaque modification and suitable stent expansion and apposition.4,10
CL is a simple technique with a short learning curve and promising initial results.11 It seems to have more effect on deep calcium than other plaque-modification techniques; therefore, it may become a standard approach for treating CCL.12 Despite successful early results in select coronary lesions,12 there are few real-world data published about the use of CL. Its safety and possible procedural complications remain unclear, and the effects of combination with other plaque-modification devices are unknown. The objective of this study was to provide further insight about the safety and efficacy of CL for the treatment of CCL, by reporting the initial outcomes of the CL by analyzing a real-world multicenter registry.
METHODSStudy design and study populationThis registry was a prospective, multicenter, single-arm study in which from August 2018 until August 2019, 57 consecutive patients with 66 calcified coronary lesions treated with the Coronary Rx Lithoplasty System (Shockwave Medical, Inc, United States) were included, following the intention-to-treat principle.
This study was conducted at 5 hospitals in a single country. All centers participated on a voluntary and unaudited basis. Eligible patients were older than 18 years with stable angina or acute coronary syndrome suitable for PCI and had CCL with significant stenosis (≥ 50% diameter stenosis) and a vessel diameter ≥ 2.5mm assessed by visual estimation and quantitative coronary angiography. The exclusion criteria in this real-world registry consisted of a target lesion located in a small vessel (<2.5mm) and the presence of significant (more than type B) coronary dissection prior to CL.
Quantitative coronary angiography was completed by an independent central core laboratory (BARCICORE Lab, Spain). The analysis was performed using dedicated coronary angiography analysis software (CAAS version 5.9, Pie Medical BV, the Netherlands). OCT data were also analyzed at the same core laboratory (BARCICORE Lab, Spain) by experienced analysts, who were also blinded to the clinical data, using proprietary offline software (LightLab Imaging, St Jude Medical Inc, United States).
This study was performed according to the provisions of the Declaration of Helsinki, ISO 14155, and clinical practice guidelines. The study protocol was approved by the Institutional Ethics Committee and the hospital's research commission. Written informed consent was obtained from all patients.
Study endpoints and definitionsThe main objectives of this study were to provide further insight about the safety and efficacy of CL for the treatment of CCL and to report short-term clinical outcomes in a very high-risk population.
Clinical and angiographic endpoints were defined according to the recommendations of the Academic Research Consortium.13 Academic Research Consortium represents a step toward standardization, facilitating the evaluation of the safety and effectiveness of devices.
Lithoplasty success was defined as achievement of <50% of residual diameter stenosis of the target lesion as assessed by visual inspection or quantitative coronary angiography, as well as successful deployment of the stent.
Bleeding as a safety endpoint was defined according to the Bleeding Academic Research Consortium.14 Major bleeding was defined as Bleeding Academic Research Consortium types 3 and 5.
Lithoplasty system and procedural characteristicsAll lesions were treated with the Coronary Rx Lithoplasty System (Shockwave Medical, Inc). The LB is a single-use angioplasty balloon catheter with a length of 12mm and available diameters ranging from 2.5mm to 4mm; it is advanced over a 0.014 inch guidewire. The LB emits acoustic circumferential pressure pulses, which allows the treatment of concentric calcified lesions (figure 1). CL allows the safe treatment of bifurcation lesions since the side-branch can be protected during the procedure. The concomitant use and order of other plaque-modification devices, such as RA and/or cutting/scoring balloon, as well as regular and special balloons (eg, semicompliant balloon, noncompliant balloon, and double-twin high-pressure balloon), were performed at the discretion of the operator. When lesion tortuosity was moderate to severe, special devices, such as guide extension catheters, were recommended.
Each procedure was performed via radial, femoral, or humeral access with a guide catheter ≥ 6 Fr. The LB was placed in the target lesion and connected to an external unit to generate pulsatile mechanical waves (figure 1). The ratio of the size of the LB to the coronary artery was 1:1. The LB was initially inflated to a pressure of 4atm and delivered 1 therapy of 10 pulses (requiring approximately 10seconds). Then, the LB was inflated to a pressure of 6atm and a total of 3 to 8 therapies (up to 80 pulses) per balloon and per lesion was delivered, with intervals of deflation to allow distal perfusion. The use of the LB is simple and requires a short learning curve, which simplifies the PCI procedure.
Due to the size of the LB, if the lesion length was >12mm, the LB was repositioned to treat the total calcified lesion. Implantation of a drug-eluting stent was performed at the discretion of the operator. Dual antiplatelet therapy was also left to the operator's discretion and initiated according to recommendations for standard of care.
Statistical analysisStatistical analysis was performed using STATA software version 15.0 (StataCorp, Tx, United States). Continuous variables are shown as mean±standard deviation (SD) or median with interquartile range [IQR]. Categorical variables are expressed as frequencies and group percentages.
RESULTSFrom August 2018 to August 2019, 57 patients (66 lesions) with CCL assessed by angiography were enrolled in the study. All lesions were treated with the Coronary Rx Lithoplasty System (Shockwave Medical, Inc). Baseline clinical, lesion, and procedural characteristics are listed in table 1, table 2, table 3, table 4, and table 5. As shown in table 1 and table 2, the study included a relatively elderly population (mean age, 72.6±9.4 years old), and high proportions of patients had diabetes (56%), and chronic kidney disease (35%). A significant percentage of patients had existing coronary heart disease (65%) and previous revascularization (44%). Most of patients also had multivessel disease (84%), and the population had a mean SYNTAX score of 23. The most frequently treated artery was the left anterior descending artery. CL was used in 30 bifurcated lesions (45%) and lateral branch protection was performed in 19 lesions (63% of the bifurcated lesions). LB was used in 8 left main coronary artery lesions. Five patients with ST-segment elevation myocardial infarction underwent CL; thromboaspiration was performed when thrombus was objectified after guidewire passage. LB was used in all cases without the presence of macroscopic thrombus.
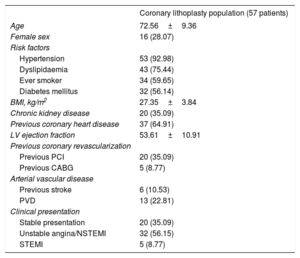
Baseline demographic and clinical characteristics (57 patients and 66 lesions)
| Coronary lithoplasty population (57 patients) | |
|---|---|
| Age | 72.56±9.36 |
| Female sex | 16 (28.07) |
| Risk factors | |
| Hypertension | 53 (92.98) |
| Dyslipidaemia | 43 (75.44) |
| Ever smoker | 34 (59.65) |
| Diabetes mellitus | 32 (56.14) |
| BMI, kg/m2 | 27.35±3.84 |
| Chronic kidney disease | 20 (35.09) |
| Previous coronary heart disease | 37 (64.91) |
| LV ejection fraction | 53.61±10.91 |
| Previous coronary revascularization | |
| Previous PCI | 20 (35.09) |
| Previous CABG | 5 (8.77) |
| Arterial vascular disease | |
| Previous stroke | 6 (10.53) |
| PVD | 13 (22.81) |
| Clinical presentation | |
| Stable presentation | 20 (35.09) |
| Unstable angina/NSTEMI | 32 (56.15) |
| STEMI | 5 (8.77) |
BMI, body mass index; CABG, coronary artery bypass graft; LV, left ventricular; NSTEMI, non–ST-elevation acute myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; STEMI, ST-segment elevation myocardial infarction.
Values are expressed as No. (%) or mean±standard deviation.
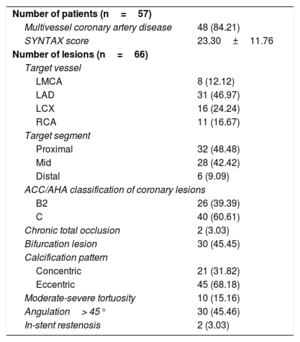
Baseline lesion characteristics (57 patients and 66 lesions)
| Number of patients (n=57) | |
| Multivessel coronary artery disease | 48 (84.21) |
| SYNTAX score | 23.30±11.76 |
| Number of lesions (n=66) | |
| Target vessel | |
| LMCA | 8 (12.12) |
| LAD | 31 (46.97) |
| LCX | 16 (24.24) |
| RCA | 11 (16.67) |
| Target segment | |
| Proximal | 32 (48.48) |
| Mid | 28 (42.42) |
| Distal | 6 (9.09) |
| ACC/AHA classification of coronary lesions | |
| B2 | 26 (39.39) |
| C | 40 (60.61) |
| Chronic total occlusion | 2 (3.03) |
| Bifurcation lesion | 30 (45.45) |
| Calcification pattern | |
| Concentric | 21 (31.82) |
| Eccentric | 45 (68.18) |
| Moderate-severe tortuosity | 10 (15.16) |
| Angulation> 45° | 30 (45.46) |
| In-stent restenosis | 2 (3.03) |
ACC/AHA, American College of Cardiology/American Heart Association; LAD, left anterior descending artery; LCX, left circumflex artery; LMCA, left main coronary artery; RCA, right coronary artery.
Values are expressed as No. (%) or mean±standard deviation.
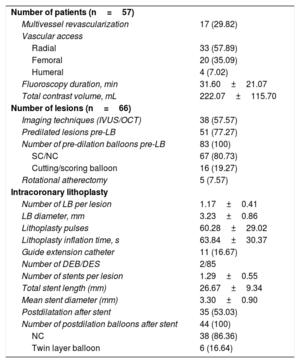
Procedural characteristics (57 patients and 66 lesions)
| Number of patients (n=57) | |
| Multivessel revascularization | 17 (29.82) |
| Vascular access | |
| Radial | 33 (57.89) |
| Femoral | 20 (35.09) |
| Humeral | 4 (7.02) |
| Fluoroscopy duration, min | 31.60±21.07 |
| Total contrast volume, mL | 222.07±115.70 |
| Number of lesions (n=66) | |
| Imaging techniques (IVUS/OCT) | 38 (57.57) |
| Predilated lesions pre-LB | 51 (77.27) |
| Number of pre-dilation balloons pre-LB | 83 (100) |
| SC/NC | 67 (80.73) |
| Cutting/scoring balloon | 16 (19.27) |
| Rotational atherectomy | 5 (7.57) |
| Intracoronary lithoplasty | |
| Number of LB per lesion | 1.17±0.41 |
| LB diameter, mm | 3.23±0.86 |
| Lithoplasty pulses | 60.28±29.02 |
| Lithoplasty inflation time, s | 63.84±30.37 |
| Guide extension catheter | 11 (16.67) |
| Number of DEB/DES | 2/85 |
| Number of stents per lesion | 1.29±0.55 |
| Total stent length (mm) | 26.67±9.34 |
| Mean stent diameter (mm) | 3.30±0.90 |
| Postdilatation after stent | 35 (53.03) |
| Number of postdilation balloons after stent | 44 (100) |
| NC | 38 (86.36) |
| Twin layer balloon | 6 (16.64) |
DEB, drug-eluting balloon; DES, drug-eluting stent; IVUS, intravascular ultrasound; LB, lithoplasty balloon; NC, noncompliant; OCT, optical coherence tomography; SC, semicompliant
Values are expressed as No. (%) or mean±standard deviation.
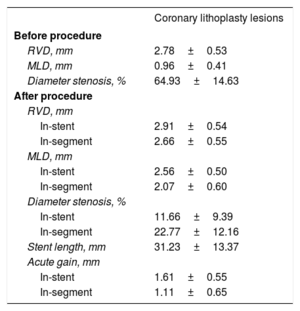
Quantitative angiographic analysis of lesions treated (n=61)
| Coronary lithoplasty lesions | |
|---|---|
| Before procedure | |
| RVD, mm | 2.78±0.53 |
| MLD, mm | 0.96±0.41 |
| Diameter stenosis, % | 64.93±14.63 |
| After procedure | |
| RVD, mm | |
| In-stent | 2.91±0.54 |
| In-segment | 2.66±0.55 |
| MLD, mm | |
| In-stent | 2.56±0.50 |
| In-segment | 2.07±0.60 |
| Diameter stenosis, % | |
| In-stent | 11.66±9.39 |
| In-segment | 22.77±12.16 |
| Stent length, mm | 31.23±13.37 |
| Acute gain, mm | |
| In-stent | 1.61±0.55 |
| In-segment | 1.11±0.65 |
MLD: minimum vessel diameter; RVD: reference vessel diameter.
Values are expressed as mean±standard deviation.
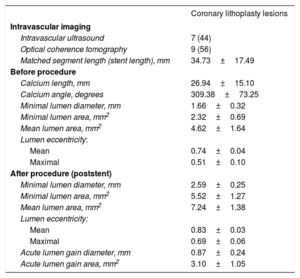
Intravascular imaging results (n=16)
| Coronary lithoplasty lesions | |
|---|---|
| Intravascular imaging | |
| Intravascular ultrasound | 7 (44) |
| Optical coherence tomography | 9 (56) |
| Matched segment length (stent length), mm | 34.73±17.49 |
| Before procedure | |
| Calcium length, mm | 26.94±15.10 |
| Calcium angle, degrees | 309.38±73.25 |
| Minimal lumen diameter, mm | 1.66±0.32 |
| Minimal lumen area, mm2 | 2.32±0.69 |
| Mean lumen area, mm2 | 4.62±1.64 |
| Lumen eccentricity: | |
| Mean | 0.74±0.04 |
| Maximal | 0.51±0.10 |
| After procedure (poststent) | |
| Minimal lumen diameter, mm | 2.59±0.25 |
| Minimal lumen area, mm2 | 5.52±1.27 |
| Mean lumen area, mm2 | 7.24±1.38 |
| Lumen eccentricity: | |
| Mean | 0.83±0.03 |
| Maximal | 0.69±0.06 |
| Acute lumen gain diameter, mm | 0.87±0.24 |
| Acute lumen gain area, mm2 | 3.10±1.05 |
Values are expressed as No. (%) or mean±standard deviation.
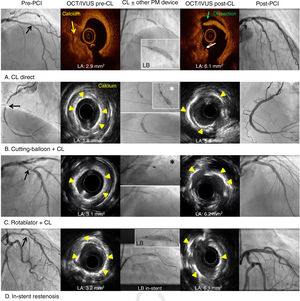
All lesions were classified as type B (39%) or C (61%) according to the American College of Cardiology/American Heart Association classification. Moreover, lesions were considered as significant calcified lesions and most of the calcium distribution was eccentric (68%). Intravascular imaging techniques (IVUS/OCT) were performed at the discretion of the operator in more than half of the patients, but only 16 cases showed minimum criteria for proper analysis to be analyzed (table 5 and figure 2).
Optical coherence tomography and intravascular ultrasound characterization of calcified lesions and the effects of coronary lithoplasty and the concomitant use of other plaque-modification devices. Black arrows represent severe calcified lesions. White asterisk represents cutting-balloon. Black asterisk represents rotablator. CL, coronary lithoplasty; IVUS, intravascular ultrasound; LA, luminal area; LB, lithoplasty balloon; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; PM, plaque-modification.
Procedural characteristics are listed in table 3. Multivessel revascularization was performed in 30% of patients. In more than half of the patients, vascular access was radial. More than 75% of lesions were predilated. It is notable that the concomitant use of other plaque-modification devices before LB was found to be safe. RA was used before the LB in 5 cases and cutting/scoring balloons were used before the LB in 16 cases. Despite use of the second generation of the device, which has improved its navigation profile to be more effective in the presence of tortuosity, in 11 cases (16.7%) a catheter extender was needed due to lesion tortuosity to place the LB at the level of the lesion.
On average, coronary lithotripsy was achieved by the use of 1 balloon [range, 1-2] delivering 3 therapies [range, 2.5-4] with a mean of 60 pulses and a total mean inflation time of 64seconds. Nine (13%) LB were broken during therapy without any dissection related to the rupture; the mean number of cycles before rupture was 3 [range, 2.5-4]. In cases where the LB was broken, the lesions had no specific characteristics, such as increased eccentricity, that could be related to the therapy.
Successful CL was achieved in 98% of cases. There was only 1 case in which it was not possible to achieve <50% of residual diameter stenosis of the target lesion after noncompliant balloon and cutting-balloon preCL; in this case, 2 LBs were applied with a total of 160 pulses, as well as 1 double-twin-layer balloon. Thus, this patient was treated with a drug-eluting balloon. The average number of stents per lesion was 1 [range, 1-2] according to lesion length and the treatment of residual stent edge dissection. Mean stent diameter was 3.3±0.9mm and total mean stent length was 26.7±9.3mm.
Quantitative angiographic characteristicsAngiographic characteristics are shown in table 4. Quantitative coronary angiography was adequately performed on 61 lesions before and after the procedure (5 cases did not show enough quality for the correct analysis). The mean reference vessel diameter was 2.8±0.5mm and lesion length was 26±10.4mm (figure 1 of the supplementary data).
Intravascular imagingIVUS/OCT results are presented in table 5. The analysis of intravascular imaging was performed a posteriori without a specific acquisition protocol: after predilating with a semicompliant balloon before the LB; and post-PCI. Before CL, calcification length on intravascular imaging was 26.9±15.1mm and minimal lumen diameter was 1.66±0.32mm. After PCI, minimal lumen diameter was 2.6±0.2mm and acute lumen gain area was 3.1±1.0 mm2.
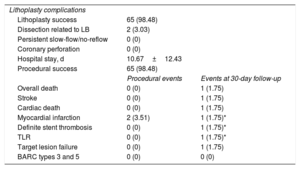
Clinical outcomes during hospital admission and at 30-day follow-upClinical events during the procedure and within 30 days after the procedure are shown in table 6. There were few complications during the procedure and there was no procedural target lesion failure and no coronary perforation or slow-flow/no-reflow. Two cases with significant dissections were successfully treated with drug-eluting stent implantation. During the hospital stay, 1 patient experienced a stroke. There was 1 death not related to the CL, an elderly patient with severe comorbidities and 3-vessel disease, who underwent successful coronary lithotripsy of right coronary artery. Two days after the procedure, the patient showed clinical instability due to the left anterior descending lesion, which was not treated during the index procedure. Given the rapid hemodynamic deterioration; according to patient and family preferences, it was decided to limit the therapeutic effort.
Angiographic, procedural, and major cardiac adverse events at follow-up (57 patients and 66 lesions)
| Lithoplasty complications | ||
| Lithoplasty success | 65 (98.48) | |
| Dissection related to LB | 2 (3.03) | |
| Persistent slow-flow/no-reflow | 0 (0) | |
| Coronary perforation | 0 (0) | |
| Hospital stay, d | 10.67±12.43 | |
| Procedural success | 65 (98.48) | |
| Procedural events | Events at 30-day follow-up | |
| Overall death | 0 (0) | 1 (1.75) |
| Stroke | 0 (0) | 1 (1.75) |
| Cardiac death | 0 (0) | 1 (1.75) |
| Myocardial infarction | 2 (3.51) | 1 (1.75)* |
| Definite stent thrombosis | 0 (0) | 1 (1.75)* |
| TLR | 0 (0) | 1 (1.75)* |
| Target lesion failure | 0 (0) | 1 (1.75) |
| BARC types 3 and 5 | 0 (0) | 0 (0) |
BARC, Bleeding Academic Research Consortium; LB, lithoplasty balloon; TLR, target lesion revascularization.
Noncumulative hierarchical adverse events.
The data are expressed as No. (%) or mean ± standard deviation.
One patient presented ST-segment elevation myocardial infarction caused by definite stent thrombosis 2 days after the procedure and underwent target lesion revascularization; OCT was performed and showed a white thrombus and a slight malapposition of the proximal edge of the stent in the mid-left anterior descending artery, which was successfully treated postdilation with a 3.5-mm noncompliant balloon. No other major cardiovascular events were reported at the 30-day follow-up.
DISCUSSIONThis is the largest real-world registry to demonstrate the feasibility, safety, and short-term efficacy of PCI for severely calcified lesions using coronary lithotripsy in an unselected and high-risk population. There are some interesting findings of this study. First, compared with historical stent registries of plaque modification for CCL,15–17 the rate of procedural complications in this registry was remarkably low. Second, LB seems to be effective for calcium rupture and facilitates stent delivery and expansion. Last, the present study is the first to demonstrate the safety of the concomitant use of other plaque-modifications devices with CL.
The use of lithoplasty balloon in calcified lesions and procedural complicationsIn calcified lesions, current plaque-modification techniques have been associated with a relatively high procedural complication rate. Complications of RA include coronary dissection, perforation, burr lodging, and slow-flow/no-reflow phenomenon.15–17 The perforation rate with rotablator varies, but nears 1%, and orbital atherectomy has a perforation rate up to 2%.15–17 Our results show a remarkably low rate of procedural complications. There were 2 dissections due to CL, but none related to the LB rupture. In our opinion, the breakage of LB seems to occur during the emission of high-energy mechanical pulses rather than during the inflation. This fact may be due to the resistance of the LB to the therapy, since in some cases there would not be rupture of the LB but rather micro-fissures or an increased porosity, which could lead to the loss of pressure.
Rotablator and orbital atherectomy generate microparticles, which may be embolized distally during the procedure causing slow-flow/no-reflow: the incidence is up to 3% with rotablator.15,16 However, calcium fragments, which are generated by LB, seem to remain in situ.10 Accordingly, in our study, there were no slow-flow/no-reflow events. Although this may be purely fortuitous, we believe it can be at least partially explained by the mechanism of this novel “special” balloon device, which seems to crack calcium circumferentially, increasing lesion compliance and facilitating stent delivery and expansion.10,11
Promising 1-month resultsSevere coronary calcification is a strong predictor of major adverse cardiovascular events (MACE) after PCI.1 Established plaque-modification techniques have shown a high MACE rate at follow-up.16,17 The ORBIT II study,17 which included 443 patients who underwent orbital atherectomy followed by stenting, showed a MACE rate of 10% at the 30-day follow-up. Our registry showed a MACE rate of 3.5% at the 30-day follow-up.
Despite the small numbers and the heterogeneous, high-risk population, our short-term results are promising and comparable to those achieved with noncalcified lesions. Our results, therefore, complement the findings of the much more selective studies using LB. The DISRUPT CAD I trial demonstrated the safety and performance of coronary lithotripsy in CCL prior to stenting with a MACE rate of 3% at the 30-day follow-up.18 The DISRUPT CAD II study, with 120 patients, demonstrated the safety of CL before stent implantation with a 30-day MACE rate of 7.6%.19 Currently, a large trial is being conducted to assess the safety and effectiveness of CL and confirm these promising results: DISRUPT CAD III with approximately 392 patients.
User-friendly plaque-modification deviceCL is a new and simple technique with a short learning curve compared with other plaque-modifications devices.11,20 Our study is the first to demonstrate the safety of the concomitant use of other plaque-modifications devices, including RA. In this high-risk population, most patients were not considered candidates for coronary artery bypass graft.
The second-generation LB showed a better crossing profile than expected for a balloon angioplasty catheter with emitters. In our registry, complex lesions (type B or C) were successfully treated despite severe calcification and eccentric calcium distribution. If moderate-to-severe tortuosity was present, LB showed feasibility with the concomitant use of a guide extension catheter.
In agreement with our initial experience and a recent case report, LB seems to facilitate expansion of in-stent restenosis due to an underexpanded stent, showing that stent struts may not impede the transmission of shockwaves to the vessel wall.21,22 Initial results of treating severe calcified lesions in the left main coronary artery with left ventricular dysfunction have been reported.23,24 Our registry included the safe and successful treatment of 8 severe calcified lesions in the left main artery with CL. The first experience with CL for the treatment of CCL in patients with ST-segment elevation myocardial infarction has been reported.25 Our registry included the successful treatment of 5 patients with ST-segment elevation myocardial infarction and severe calcified lesions with CL.
Some interesting technical tips should be highlighted. CL may be effective in large vessels, since LB are available in diameters up to 4mm, which is larger than rotablator burrs and orbital crowns. LB may also be a feasible treatment for bifurcated lesions since it allows the placement of 2 wires during the procedure. Intravascular imaging techniques should be performed for CL guidance and to achieve optimal PCI results; our study showed 1 stent thrombosis caused by stent malapposition, which may have been avoided by performing OCT.
LimitationsThis was an observational study, not a randomized controlled study. The sample size was relatively small, with a short follow-up time, absence of a comparison group, with heterogeneity of lesions included and with self-reporting of events. However, larger registries with longer follow-up periods are needed to investigate the use of CL and to assess future randomized studies comparing CL in severe calcified lesions to standard treatment. It is important to understand the effect of prolonged balloon inflation related to silent ischemia and the effect of delivering intermittent high-pressure pulses on vessel walls in terms of inflammation and stent restenosis in long-term follow-up. Additionally, despite the rupture of LB, the procedure seems to be safe, although rupture is unpredictable and always occurs while applying the therapy, which requires the use of another LB and increases the cost. Finally, routine pre- and postintervention IVUS/OCT were not performed to predefine inclusion parameters or to confirm optimal stent results.
ConclusionsThis is a real-world multicenter registry, which supports the feasibility, safety, and short-term efficacy of PCI for calcified lesions using CL in an unselected and high-risk population with promising results. In addition, the present study is the first to demonstrate the safety of the concomitant use of other plaque-modification devices with LB. Larger multicenter registries with long-term follow-up are required to clarify the role of plaque modification using CL.
CONFLICTS OF INTERESTAll the authors state that there are no potential conflicts of interest related to this work.
- -
Severe coronary calcium increases the complexity of percutaneous revascularization. Available techniques for the treatment of severe calcified lesions can be classified as nonballoon technology (rotablator, orbital atherectomy, and excimer laser) or balloon-based technology (CL).
- -
Coronary lithotripsy is a simple technique with a short learning curve: it may increase lumen diameter and lesion compliance. Despite successful early results in select coronary lesions, there are few real-world data published about coronary lithotripsy, and its safety and possible procedural complications remain unclear.
- -
This is a real-world multicenter registry, which supports the feasibility, safety, and short-term efficacy of CL for calcified lesions in an unselected and high-risk population.
- -
This study is the first to demonstrate the safety of the concomitant use of other plaque-modification devices with CL. A LB is a user-friendly device, which seems to be safe and effective for complex lesions, and has a remarkably low rate of procedural complications.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.02.010