Peritoneal dialysis has been proposed as a therapeutic alternative for patients with refractory congestive heart failure. The objective of this study was to assess its effect on long-term clinical outcomes in patients with advanced heart failure and renal dysfunction.
MethodsA total of 62 patients with advanced heart failure (class III/IV), renal dysfunction (glomerular filtration<60mL/min/1.73 m2), persistent fluid congestion despite loop diuretic treatment and at least 2 previous hospitalizations for heart failure were invited to participate in a continuous ambulatory peritoneal dialysis program. Of these, 34 patients were excluded and adjudicated as controls. The most important reasons for exclusion were refusal to participate, inability to perform the technique and abdominal wall defects. The primary endpoint was all-cause mortality and the composite of death/readmission for heart failure. To account for baseline imbalance, a propensity score was estimated and used as a weight in all analyses.
ResultsThe peritoneal dialysis (n=28) and control groups (n=34) were alike in all baseline covariates. During a median follow-up of 16 months, 39 (62.9%) died, 21 (33.9%) patients were rehospitalization for heart failure, and 42 (67.8%) experienced the composite endpoint. In the propensity score-adjusted models, peritoneal dialysis (vs control group) was associated with a substantial reduction in the risk of mortality using complete follow-up (hazard ratio=0.40; 95% confidence interval, 0.21-0.75; P=.005), mortality using days alive and out of hospital (hazard ratio=0.39; 95% confidence interval, 0.21-0.74; P=.004) and the composite endpoint (hazard ratio=0.32; 95% confidence interval, 0.17-0.61; P=.001).
ConclusionsIn refractory congestive heart failure with concomitant renal dysfunction, peritoneal dialysis was associated with long-term improvement in clinical outcomes.
Keywords
Systemic congestion commonly occurs in patients with advanced heart failure (HF) and is considered a hallmark in those with acute heart failure (AHF).1 In addition, there is strong evidence suggesting that congestion may play an important role in progression of the disease.2, 3, 4 Indeed, recent data support the role of fluid retention in the pathogenesis of renal dysfunction (cardiorenal syndrome) and subsequent diuretic resistance,5, 6 which are associated with limited therapeutic options7, 8 and poor prognosis.9, 10, 11 In this context, 2 related procedures have been proposed for the management of these patients: a) intermittent ultrafiltration, which is particularly useful during episodes of acute decompensation,12, 13 and b) continuous ambulatory peritoneal dialysis (CAPD), which has been considered an attractive alternative for the treatment of refractory congestive heart failure (CHF) by offering a continuous and more physiological ultrafiltration process.14, 15, 16, 17, 18, 19, 20, 21 Indeed, our group, as well as other groups, have described patient improvement in clinical and functional status, favorable changes in echocardiographic and hemodynamic parameters, and reduction in hospitalization rates associated with the use of CAPD with an acceptable rate of adverse effects.14, 15, 16, 17, 18, 19, 20, 21 Nevertheless, the effect of CAPD on long-term clinical outcomes is still unknown.
The aim of this study was to compare clinical outcomes between patients included in a CAPD program vs a similar cohort of CHF patients who were eligible for CAPD but who refused to be enrolled or were excluded from the program.
Methods Study Group and ProtocolWe prospectively studied a cohort of 62 patients, who were followed up in the HF unit of the Hospital Clínico Universitario de Valencia from August 1, 2008 to June 1, 2011, and who met the following inclusion criteria: a) at least 2 prior admissions for AHF, with the last episode being in the past 6 months; b) New York Heart Association (NYHA) functional class III/IV; c) persistent congestion despite optimal loop-diuretic therapy, and d) the presence of renal dysfunction documented at least once in the last 12 months (estimated glomerular filtration rate [eGFR]<60mL/min/1.73 m2).
AHF was defined as a rapid onset of symptoms and signs secondary to abnormal cardiac function and the presence of objective evidence of structural or functional abnormality of the heart at rest (such as cardiomegaly, third heart sound, cardiac murmur, an abnormality demonstrated by an echocardiogram or raised natriuretic peptides).7, 8
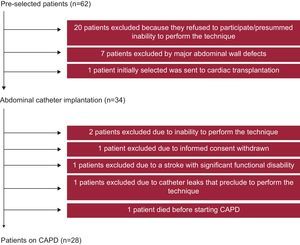
During their last hospitalization, patients who met the inclusion criteria (n=62) were invited to participate in the CAPD program as an alternative therapeutic option for the relief of fluid overload. Of these 62 patients, 20 refused to participate or showed inability to perform the technique at home, 7 were excluded because of the presence of abdominal wall defects, and 1 patient, who was initially selected, was subsequently withdrawn to undergo a cardiac transplantation (Figure 1). Therefore, an abdominal catheter was surgically implanted in the remaining 34 patients, at a median of 31 days [interquartile range, 2-37] since the last hospitalization. Subsequently, 6 patients did not start dialysis for various reasons listed in Figure 1. Finally, we were able to initiate CAPD in 28 patients, at a median of 58 days [40-64] since the abdominal catheter implantation. The CAPD program consisted of 2-3 times/day exchange with dialysate solution (1.36%–2.27% of glucose), the latter titrated according to the patient's response. Our protocol followed the current international guidelines on the treatment of peritoneal dialysis-related infections,22, 23 and peritoneal access.24
Figure 1. Flow chart. CAPD, continuous ambulatory peritoneal dialysis.
Demographic information, medical history, vital signs, 12-lead electrocardiogram, echocardiography, laboratory data and pharmacological treatments were routinely assessed using pre-established questionnaires. Concomitant use of medications for the treatment of HF was individualized according to established guidelines,7, 8 and all patients (including controls) received a similar regimen of follow-up visits. According to the protocol, the loop-diuretic dosage was not initially modified until a clinical reduction of systemic congestion was verified. The protocol was approved by the ethical committee of our center, and was in accordance with the principles of the Declaration of Helsinki and national regulations.
Treatment InterventionOnly those patients who fulfilled the inclusion criteria and underwent the CAPD procedure constituted the active treatment group (n=28). Patients who also fulfilled the inclusion criteria but were not finally enrolled in the CADP program were assigned as controls (n=34). The control group was managed according to established treatment guidelines.7, 8
EndpointsThe primary endpoint was all-cause mortality (using complete follow-up and days alive and out of hospital [DAOH] as follow-up time) and the composite of death/readmission for AHF.
Statistical AnalysisData analysis was performed according to the statistical analysis plan developed by Cuore International, Inc. (Scottsdale, Arizona, United States). Continuous variables were expressed as mean (1 standard deviation) or median [nterquartile range] when appropriate. Discrete variables are shown as percentages.
To estimate the causal effects of CAPD, a propensity score (PS) weighting was estimated using a boosted CART algorithm25 implemented in R (the twangTWANG package).26 The variables included in the PS-weighting are listed in Table 1. We used 20 000 iterations, a shrinkage parameter of 0.0005, and a stopping rule that minimizes the mean of the Kolmogorov-Smirnov test statistics. To reduce the type I error by preserving the sample size, the PS was stabilized according to: if CAPD=1, then sPS=p/PS, and if CAPD=0, then sPS=(1–p)/(1–PS), where p is the probability of treatment without considering covariates.27 The performance of the PS was evaluated through the calculation of the standardized effect size, and by examining the spread of the PS among the treatment and comparison groups.26 The PS was then incorporated as weights into a regression model with only the treatment as a predictor variable and no covariates. As recommended, the 95% confidence intervals (95%CIs) were estimated using “robust” standard errors (also known as the “Huber sandwich estimator”).27
Table 1. Baseline Characteristics Comparison Before and After Propensity Score-weighting
| Statistics for assessing balance | ||||||
| Un-weighted | Weighted | |||||
| Variables | STD effect SZ | KS | KS P-value | STD effect SZ | KS | KS P-value |
| Age, years | −0.129 | 0.233 | .260 | −0.094 | 0.205 | .405 |
| Male | −0.034 | 0.015 | .921 | 0.011 | 0.005 | .975 |
| Weight, kg | 0.054 | 0.179 | .578 | 0.026 | 0.175 | .602 |
| Reason for last hospitalization | ||||||
| ADHF | 0.139 | 0.057 | .601 | 0.193 | 0.078 | .466 |
| Pulmonary edema | −0.268 | 0.099 | .302 | −0.348 | 0.128 | .180 |
| Hypertensive-related | 0.284 | 0.036 | .305 | 0.313 | 0.039 | .152 |
| Shock | 0.036 | 0.006 | .869 | 0.057 | 0.01 | .717 |
| Hypertension | 0.299 | 0.082 | .255 | 0.287 | 0.078 | .283 |
| Dyslipidemia | 0.425 | 0.197 | .103 | 0.347 | 0.161 | .171 |
| Diabetes mellitus | 0.272 | 0.137 | .291 | 0.184 | 0.093 | .482 |
| Diabetes mellitus, insulin-dependent | 0.206 | 0.099 | .419 | 0.111 | 0.053 | .674 |
| Current smoker | 0.050 | 0.013 | .889 | 0.028 | 0.007 | .964 |
| Previous smoker | −0.303 | 0.149 | .241 | −0.278 | 0.137 | .283 |
| Alcohol abuse | −0.106 | 0.023 | .713 | −0.113 | 0.025 | .619 |
| Etiology | ||||||
| Ischemic heart disease | 0.149 | 0.061 | .886 | 0.116 | 0.067 | .856 |
| Valvular heart disease | 0.143 | 0.069 | .581 | 0.190 | 0.092 | .461 |
| History of MI | 0.128 | 0.065 | .610 | 0.057 | 0.029 | .817 |
| History of Stroke | −0.033 | 0.011 | .946 | −0.078 | 0.025 | .732 |
| History of PAD | 0.262 | 0.116 | .300 | 0.172 | 0.076 | .510 |
| History of renal failure | 0.012 | 0.034 | .844 | −0.049 | 0.044 | .741 |
| History of COPD | 0.259 | 0.109 | .312 | 0.240 | 0.101 | .354 |
| Peripheral edema | 0.195 | 0.069 | .449 | 0.201 | 0.072 | .426 |
| Pleural effusion | 0.522 | 0.25 | .043 | 0.473 | 0.226 | .071 |
| NYHA Class III | −0.316 | 0.078 | .244 | −0.327 | 0.08 | .251 |
| Charlson comorbidity index | 0.247 | 0.197 | .252 | 0.161 | 0.162 | .445 |
| Heart rate, bpm | 0.100 | 0.227 | .262 | 0.056 | 0.189 | .462 |
| Systolic blood pressure, mmHg | 0.050 | 0.174 | .559 | −0.006 | 0.15 | .734 |
| Cardiac rhythm | ||||||
| Sinus rhythm | 0.450 | 0.225 | .081 | 0.434 | 0.217 | .099 |
| Atrial fibrillation | −0.263 | 0.126 | .313 | −0.222 | 0.106 | .390 |
| Atrial flutter | −0.108 | 0.023 | .725 | −0.137 | 0.029 | .532 |
| Type of BBB | ||||||
| None | 0.308 | 0.153 | .231 | 0.208 | 0.103 | .421 |
| Complete LBBB | −0.457 | 0.221 | .073 | −0.410 | 0.198 | .106 |
| Complete RBBB | 0.263 | 0.097 | .324 | 0.345 | 0.127 | .185 |
| Pacemaker rhythm | −0.464 | 0.187 | .074 | −0.402 | 0.162 | .119 |
| Hemoglobin, g/dL | −0.233 | 0.105 | .959 | −0.241 | 0.103 | .964 |
| WBC counts, ×106 cells/μL | 0.050 | 0.17 | .606 | 0.025 | 0.152 | .744 |
| Neutrophils, ×106 cells/μL | −0.018 | 0.158 | .700 | −0.028 | 0.161 | .676 |
| Lymphocytes, ×106 cells/μL | 0.315 | 0.288 | .101 | 0.226 | 0.233 | .269 |
| Serum creatinine, mg/dL | 0.217 | 0.193 | .465 | 0.143 | 0.171 | .632 |
| eGFR, mL/min per 1.73 m2 | 0.040 | 0.21 | .395 | 0.155 | 0.193 | .502 |
| Sodium, mEq/L | 0.122 | 0.208 | .299 | 0.081 | 0.204 | .321 |
| NT-proBNP, pg/mL | −0.217 | 0.181 | .567 | −0.205 | 0.184 | .538 |
| CA125, U/mL | 0.024 | 0.214 | .360 | 0.003 | 0.215 | .370 |
| LVEF, % | −0.064 | 0.139 | .808 | −0.045 | 0.144 | .766 |
| On beta-blockers | −0.164 | 0.082 | .526 | −0.144 | 0.072 | .559 |
| Diuretics | ||||||
| On ACE inhibitor | 0.070 | 0.036 | .779 | 0.099 | 0.05 | .694 |
| On ARB | 0.050 | 0.013 | .886 | 0.057 | 0.014 | .828 |
| ICD | −0.232 | 0.092 | .378 | −0.170 | 0.068 | .505 |
| CRT | −0.237 | 0.076 | .375 | −0.173 | 0.055 | .496 |
ACE, angiotensin converting enzyme; ADHF, acute decompensated heart failure; ARB, angiotensin II receptor blockers; BBB, bundle branch block; CA125, antigen carbohydrate 125; COPD, chronic pulmonary obstructive disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter defibrillator; KS, Kolmogorov-Smirnov test statistic; KS P-value, Kolmogorov-Smirnov associated P-value; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York heart association; PAD, peripheral arterial disease; RBBB, right bundle branch block; STD effect SZ, standardized effect size; WBC, white blood cell count.
For all survival analyses, patient follow-up was censored if death or cardiac transplantation occurred during the follow-up period.
The cumulative risk for all-cause mortality (using complete follow-up and DAOH) and for the composite endpoint of mortality and AHF rehospitalization were depicted using the Kaplan-Meier method, and their differences were tested by the Cox test. The effect of the intervention was assessed by estimating a PS-weighted hazard ratio (HR) through a fitted Cox regression.
PS-weighted absolute risk differences and their reciprocal (number needed to treat) for the CAPD group were estimated from the Cox analysis, and for each clinical endpoint.28 As recommended, these estimates were calculated at specific time points during the follow-up.29 A 2-sided P-value of<.05 was considered to be statistically significant for all analyses. All analyses were performed using STATA 12.0 and R.
ResultsAs part of the inclusion criteria, all patients were in NYHA class III/IV, had previous admissions for AHF and showed persistent signs and symptoms of congestion (despite treatment with loop diuretics). The mean age was 73.4 (9.2) years; 75.8% were men, and 66.1% had a prior history of ischemic heart disease. The medians for Charlson comorbidity index, eFGR, left ventricular ejection fraction, plasma N-terminal pro-brain natriuretic peptide and daily furosemide dose were 4 [3-6], 30.6mL/min/1.73 m2 [20-44.5], 38.5% [30%-49%], 10 703 pg/mL [5672-23 075] and 160mg [120-160], respectively. No significant differences were observed among clinical, electrocardiographic, laboratory and medical treatment between CAPD and control patients (Table 2). The weighted comparison in baseline covariates showed an excellent balance between the 2 CAPD treatment arms (Table 1).
Table 2. Baseline Characteristics
| Control group (n=34) | CAPD patients (n=28) | P | |
| Demographic and medical history | |||
| Age, years | 77 [69-79] | 75 [68-78] | .322 |
| Male | 26 (76.5) | 21 (75) | 1 |
| Weight | 80 [66-90] | 76 [70-86] | .994 |
| Hypertension | 30 (88.2) | 27 (96.4) | .366 |
| Dyslipidemia | 20 (58.8) | 22 (78.6) | .112 |
| Diabetes mellitus | 10 (29.4) | 11 (39.3) | .434 |
| Current smoker | 2 (5.9) | 2 (7.1) | 1 |
| Previous smoker | 16 (47.1) | 9 (32.1) | .301 |
| Ischemic heart disease | 22 (64.7) | 19 (67.9) | 1 |
| Valvular heart disease | 11 (32.3) | 11 (39.3) | .604 |
| COPD | 6 (17.6) | 8 (28.6) | .368 |
| Peripheral edema | 28 (82.3) | 25 (89.3) | .494 |
| NYHA Class III-IV | 34 (100) | 28 (100) | 1 |
| Charlson comorbidity index | 4 [3-6] | 4.5 [4-5.5] | .334 |
| Vital signs | |||
| Heart rate, bpm | 76 [65-90] | 79 [72-100] | .419 |
| SBP, mmHg | 120 [112-140] | 129 [111-150] | .656 |
| DBP, mmHg | 70 [60-80] | 70 [61-80] | .814 |
| Electrocardiography | |||
| Atrial fibrillation | 18 (52.9) | 10 (35.7) | .207 |
| QRS>120 ms | 21 (61.8) | 13 (46.4) | .306 |
| LBBB | 16 (47.1) | 7 (25) | .113 |
| Laboratory | |||
| Hemoglobin, g/dL | 11 [9.8-12.8] | 11.1 [9.7-12.6] | .692 |
| Serum creatinine, mg/dL | 2.15 [1.42-2.78] | 2.22 [1.64-3.27] | .432 |
| Urea, mg/dL | 97 [76-140] | 106 [67-145] | .882 |
| eGFR, a mL/min per 1.73 m2 | 31 [22-49] | 30 [18-39] | .404 |
| Sodium, mEq/L | 137 [135-141] | 139 [136-142] | .259 |
| NT-proBNP, pg/mL | 10 703 [5672-34 837] | 10 565 [5506-18 985] | .733 |
| CA125, U/mL | 60 [31-145] | 86 [40-142] | .515 |
| Echocardiography | |||
| LVEF, % | 39 [29-51] | 37 [31-46] | .887 |
| LVDD, mm | 60 [53-72] | 60 [50-66] | .213 |
| PASP, b mmHg | 47 [38-56] | 52 [42-63] | .206 |
| Medical treatment and devices | |||
| Beta-blockers | 20 (58.8) | 16 (57.1) | 1 |
| Furosemide dosage, mg | 160 [120-160] | 150 [120-180] | .922 |
| Thiazide | 3 (8.8) | 2 (7.1) | 1 |
| Spironolactone | 14 (41.2) | 9 (32.1) | .599 |
| ACEI | 11 (32.3) | 8 (28.6) | .788 |
| ARB | 6 (17.6) | 6 (21.4) | .755 |
| Statins | 17 (50) | 20 (71.4) | .120 |
| Oral anticoagulants | 15 (44.2) | 14 (50) | .799 |
| Nitrates | 10 (29.4) | 8 (28.6) | 1 |
| Digoxin | 4 (11.8) | 5 (17.9) | .719 |
| Pacemaker | 10 (29.4) | 3 (10.7) | .116 |
| ICD | 8 (23.5) | 4 (14.3) | .521 |
| CRT | 5 (14.7) | 2 (7.14) | .442 |
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; CA125, antigen carbohydrate 125; CAPD, chronic ambulatory peritoneal dialysis; COPD, chronic pulmonary obstructive disease; CRT, cardiac resynchronization therapy; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter defibrillator; LBBB, left bundle branch block; LVDD, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary arterial systolic pressure; SBP, systolic blood pressure.
Data are expressed as median [interquartile range] or no. (%).
a Using Modification of Diet in Renal Disease formula.
b Data available in 51 patients.
Among the 62 patients initially eligible for participation in the CAPD program, there were 39 deaths (62.9%), 21 readmissions for AHF (33.9%) and 42 deaths/readmissions for AHF (67.8%) during the follow-up (at a median of 16 months [6-22]). The cause of death was identified as cardiovascular in 31 (79.5%); of these, the cause of death was classified as death secondary to progressive HF in 18 patients.
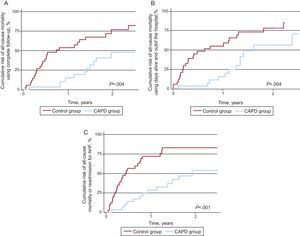
When stratified by treatment intervention, patients who underwent CAPD showed lower rates of: a) death using the entire follow-up (2.81 vs 7.34 per 10 patients-year of follow-up; P=.004); b) death using DAOH (3.55 vs 9.50 per 10 patients-year of follow-up; P=.004); c) readmission for AHF (2.13 vs 5.99 per 10 patients-year of follow-up; P=.011), and d) the composite endpoint of death/readmission for AHF (3.57 vs 10.91 per 10 patients-year of follow-up; P<.001). Substantial differences for all-cause mortality using the entire follow-up (Figure 2A), DAOH (Figure 2B) and the composite of death/readmission for AHF (Figure 2C) were observed as early as the first months, and reached their maximum around 1 year. Moreover, patients in the active treatment group showed lower rates of death from cardiovascular causes compared to controls: 8 vs. 23 patients, P<.001, especially ascribed to a substantial decrease in death due to HF progression (4 vs 14 patients; P=.004). No significant differences were observed of deaths from non-cardiovascular causes (3 vs 5 patients; P=.493). Among patients on CAPD, notably, only 4 deaths in the CAPD group were attributed to progressive HF while 2 of the deaths registered were due to complicated peritonitis.
Figure 2. Cumulative incidence of all-cause mortality and the composite endpoint of all-cause mortality or readmission for acute heart failure stratified by continuous ambulatory peritoneal dialysis therapy therapy. A: All-cause mortality using complete follow-up. B: All-cause mortality using days alive and out of hospital as follow-up time. C: All-cause mortality or readmission for acute heart failure. AHF, acute heart failure; CAPD, continuous ambulatory peritoneal dialysis.
Propensity Score-weighted AnalysesTable 3 shows the treatment-associated PS-weighted HR for each endpoint. Patients on CAPD displayed a significant risk reduction in all clinical endpoints as compared to the control group. The amount of risk reduction varied from 60%-70%, except for cardiovascular-related death, which showed a reduction close to 80%.
Table 3. Continuous Ambulatory Peritoneal Dialysis and Clinical Endpoints. Adjusted Risks
| HR (95%CI) | P | |
| All-cause death | ||
| Complete follow-up | 0.40 (0.21-0.75) | .005 |
| Days alive and out of hospital | 0.39 (0.21-0.74) | .004 |
| Cardiovascular death | 0.18 (0.04-0.74) | .017 |
| Progressive heart failure death | 0.29 (0.10-0.86) | .026 |
| Combined all-cause death and rehospitalization for AHF | 0.32 (0.17-0.61) | .001 |
95%CI, 95% confidence interval; AHF, acute heart failure; HR, hazard ratio.
By emulating an intention-to-treat analysis, in which all patients who underwent peritoneal catheter implantation (n=34), and regardless of whether CAPD was started or not, we found that the adjusted HRs for clinical endpoints also pointed toward a prognostic benefit of CAPD. These differences were important in magnitude but did not reach statistical significance for mortality using complete follow-up (HR=0.62; 95%CI, 0.32-1.20; P=.155) or mortality using DAOH (HR=0.63; 95%CI, 0.32-1.22; P=.167) but significant for the composite of death/readmission for AHF (HR=0.52; 95%CI, 0.28-0.98; P=.042).
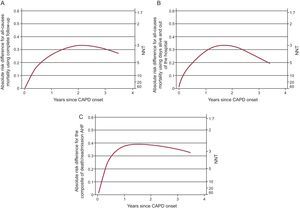
As an absolute measure of the association between CAPD and all-cause mortality, the PS-adjusted absolute risk differences and number needed to treat were estimated and depicted graphically over the follow-up time (Figure 3). Evaluated at 1 year, we needed to treat 3-5 patients with CAPD in order to prevent 1 death or composite endpoint of death/readmission for AHF. Maximum benefits for all the outcomes were observed between the first and second year after CAPD onset.
Figure 3. Absolute risk reductions and number needed to treat for time-to-event outcomes. A: All-cause mortality using complete follow-up. B: All-cause mortality using days alive and out of hospital as follow-up time. C: All-cause mortality or readmission for acute heart failure. AHF, acute heart failure; CAPD, continuous ambulatory peritoneal dialysis; NNT, number needed to treat.
DiscussionThe results of this study indicate that CAPD may play a significant role in modifying the natural history of patients with refractory CHF, in which persistent fluid overload (despite intensive diuretic therapy) and the coexistence of renal failure is also present. Indeed, the magnitude of the mortality reduction attributed to CAPD was striking in terms of relative and absolute risk reductions. A relative risk reduction of more than 50% was observed for all of the clinical endpoints, findings that were aligned with an estimated number needed to treat ranging from 3-6. Using the same cohort, our group recently showed evidence indicating that CAPD was associated with a significant and marked improvement in NYHA class, physical performance (distance walked in 6min), quality of life (Minnesota Living With Heart Failure Questionnaire), and biochemical profile at 45 days and 180 days.20 Notably, other groups have reported similar findings, in line with CAPD improving surrogate endpoints.14, 15, 16, 17, 18, 19 However, there are no data on the effect of CAPD on major clinical outcomes, perhaps because of the difficulty of selecting an appropriate comparison group.
To the best our knowledge, we believe this is the first study to make a formal prognostic comparison with a control group. In addition, unlike other series, our population included a non-selected population with CHF and CAPD was indicated for cardiac indications.
Ultrafiltration in Heart FailureDuring the last few decades, extracorporeal ultrafiltration has been used to remove fluid from diuretic-refractory hypervolemic patients. Recent trials using user-friendly machines have been shown to be effective for decongestion of patients with fluid overload.12, 13 For instance, in patients with decompensated HF, the UNLOAD (The randomized Ultrafiltration vs IV Diuretics for Patients Hospitalized for Acute Decompensated CHF) trial showed that ultrafiltration safely produces greater short-term weight and fluid loss than intravenous diuretics.30 In addition, the group assigned to ultrafiltration showed fewer rehospitalizations at 90 days but failed to demonstrate a survival benefit.30 In addition, the implementation of this technique requires specialized training, equipment and monitoring, limiting this approach to specific units during episodes of decompensation. Additionally, certain safety and economic issues are still a cause of concern.12, 13
Peritoneal dialysis is a renal replacement therapy that has emerged as a therapeutic alternative for fluid overload control in patients with refractory CHF, offering a possibility of slow, daily and ambulatory ultrafiltration.
Previous StudiesOur results are consistent with various case reports and some observational studies showing the beneficial effect of CAPD on clinical, hemodynamic, biochemical and/or echocardiographic parameters.14, 15, 16, 17, 18, 19 However, most of these studies were retrospective and did not clearly define the inclusion criteria, and most of the patients included exhibited end-stage renal failure. For instance, in one of the larger studies, Gotloib et al.,14 found a significant clinical and hemodynamic improvement 1-year after peritoneal dialysis onset in a sample of 20 patients with end-stage CHF and mean GFR=14.84 (3.8)mL/min. In contrast, patients in our cohort exhibited evidence of renal dysfunction between stages 2-4 (median eGFR=30mL/min/1.73 m2), at which renal replacement therapies are not currently indicated. Likewise, a recent study performed in Spain (similar to our cohort concerning GFR) reported a marked clinical (NYHA) and hemodynamic (pulmonary artery pressure) improvement associated with CAPD in 17 patients with refractory CHF.16 In addition, these authors found similar mortality rates (life expectancy of 82% after 12 months of treatment and of 70% and 56% after 18 and 24 months, respectively) to those observed in this work (Figure 2A). Finally, these authors reported that peritoneal dialysis was cost-effective compared with the standard treatment.16 Recently, in 118 patients with refractory CHF included in a peritoneal dialysis program, Koch et al.21 reported that survival rates after 3, 6, and 12 months were 77%, 71%, and 55%.
Concerning the safety of this procedure, we previously reported an elevated rate of peritonitis (1 episode every 16.18 months) as compared with other contemporary large series of subjects on peritoneal dialysis22 but similar to those observed in cardiorenal patients.14 We believe that this finding may be attributed in part to the elevated age of our cohort, which translates into higher comorbidity scores. However, if we look at the poor prognosis in these patients (Figure 2), the risk of peritonitis associated with the procedure seems to be acceptable, in particular when well-established therapies for these patients are absent.7, 8
PathophysiologyRecent evidence has highlighted the role of congestion, not only as a marker of HF severity, but also as a surrogate for complex interactions involving systemic, cardiac, renal and neurohormonal activation, processes that ultimately promote the progression of the disease.4 For instance, the following mechanisms have been proposed as playing an important role in the pathophysiology of systemic congestion and HF progression1, 2, 3, 4, 5: a) neurohormonal activation (favoring sodium retention); b) decreased renal filtration secondary to renal venous congestion; c) predisposition to subendocardial cardiac ischemia; d) architectural ventricular modifications; e) endotoxin translocation, and f) endothelial interactions.
Along this line, we reported a substantial reduction in surrogate markers indicative of systemic and renal venous congestion in patients on CAPD.20, 31 Similarly, at 6 months after the start of CAPD, all patients in this study on CAPD, except one, tolerated furosemide reduction to 80mg/day, following a median starting dose of 160mg. Whether the prognostic effect attributable to CAPD in refractory CHF patients is limited to fluid overload control or there is an additional pleiotropic effect (such as clearance of inflammatory mediators) remains to be clarified.32
Logistic IssuesIn this article we showed that CAPD may be considered a feasible alternative for the treatment of CHF patients in daily practice. Indeed, CAPD was initiated in up to 82% of patients in which an abdominal catheter was implanted. From a logistic perspective, CAPD offers some advantages over other ultrafiltration techniques, the most important being: slow and daily ambulatory ultrafiltration, simplicity (the procedure is easily carried out), preservation of residual renal function, and hemodynamic stability.
LimitationsThe main limitation of this study stems from the fact that it is a small, single center observational study. Nevertheless, we believe that our results are sufficiently robust to have clinical significance. Even though the intervention was not randomly allocated, the control group shared similar baseline characteristics with the CAPD group, since both groups met the inclusion criteria; moreover, the use of PS-weighted regression ensured that both groups were comparable at least in all measured confounders. A randomized clinical trial in this setting would be difficult for ethical reasons, in particular owing to the difficulty of blinding the patients and the investigator to the treatment intervention.33
However, due to the scarce information on the efficacy, tolerability and safety of CAPD in this population of HF patients, we believe that further studies are warranted to confirm our results and to define the optimal profile of candidates for this technique and the optimal technique-logistic approach.
ConclusionsIn this observational study, we found that the risk of major outcomes was significantly reduced in patients with advanced and refractory CHF and concomitant renal dysfunction who underwent CAPD. Additional studies, hopefully in more controlled scenarios, are needed to confirm these results and to define the clinical utility of this technique in this challenging subset of HF patients.
FundingThis study was supported by unrestricted grants from the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III, RED HERACLES RD06/0009/1001 (Madrid, Spain), help for projects of emerging groups in 2010 of the Conselleria de Sanitat de Valencia (DOCV 6.175, 30/12/2009-Annex III ), Spanish Society of Cardiology (Beca Esteve 2009) and Fresenius Medical Care.
Conflicts of interestNone declared.
Received 14 March 2012
Accepted 6 May 2012
Corresponding author: Servicio de Cardiología, Hospital Clínico Universitario, Avda. Blasco Ibáñez 17, 46010 Valencia, Spain. yulnunez@gmail.com