To analyze survival in heart failure (HF) patients treated at a specialized unit.
MethodsProspective cohort-based study of HF patients treated at a specialized unit from 2011 to 2017. Observed 1- and 3-year mortality rates were compared with those predicted by the Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score.
ResultsWe studied 1280 patients, whose median MAGGIC risk score was 19 [interquartile range, 13-24]. Prescription rates of beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, mineralocorticoid receptor antagonists, and sacubitril-valsartan were 93%, 67%, 22%, 73%, and 16%, respectively. The MAGGIC risk score showed good discrimination for mortality at 1 year (c-statistic=0.71) and 3 years (c-statistic=0.76). Observed mortality was significantly lower than predicted mortality, both at 1 year (6.2% vs 10.9%; observed/predicted ratio=0.57; P<.001) and at 3 years (16.7% vs 27.7%; observed/predicted ratio=0.60; P<.001). This discrepancy was found in several subgroups, except in patients aged> 70 years (29.9% vs 34.7%; observed/predicted ratio=0.86; P=.126) and in patients with ejection fraction> 40% (19.6% vs 20.7%; observed/predicted ratio=0.95; P=.640).
ConclusionsMortality in HF patients treated at a specialized clinic was significantly lower than that predicted by the MAGGIC risk score.
Keywords
Research in recent decades has shown that various drugs and devices can reduce morbidity and mortality in patients with heart failure (HF) and that these treatments are particularly effective in patients with a reduced left ventricle ejection fraction (LVEF <40%).1 Even patients with refractory HF can benefit from more advanced treatment options such as heart transplant and ventricular assist devices, which can improve both prognosis and quality of life.2
Despite the therapeutic advances that have been achieved, hospitalization and mortality rates remain high among HF patients in the “real world”.3 Contributory factors include barriers to the close monitoring and optimal treatment required by these patients. Promotion of dedicated HF care programs and units can help overcome these barriers. Programs of this type have been found to significantly reduce hospital readmission rates and improve survival, albeit more modestly.4
The Spanish Society of Cardiology (SEC) has established a series of quality assurance requirements that must be met by HF units to receive certification within its excellence program for 3 types of units: community units, specialized units, and advanced units.5 The SEC-Excellence program uses different clinical indicators to monitor the quality of care delivery at certified units. Readmission and mortality rates are among the main indicators.
The aim of this study was to analyze mortality in a cohort of HF patients treated at a SEC-certified unit and compare rates with those calculated using a widely validated multivariable scoring system for predicting mortality in HF.6
MethodsStudy descriptionObservational cohort study of patients with HF referred to the HF unit at our hospital for the first time between January 1, 2011 and December 31, 2017. Data were obtained from the SiMon clinical record management system, a purpose-built application designed by the hospital's computer department that includes a tabulated database containing prospectively collected data. Patients provided informed consent permitting us to use their clinical data for research purposes.
Study settingThe study setting was the HF unit at the Cardiology Department of Complejo Hospitalario Universitario A Coruña in Galicia, Spain. This unit is the referral unit for HF patients in the health care district of A Coruña, which has approximately 550 000 inhabitants. It also receives patients with refractory HF from other hospitals in the autonomous community of Galicia (≈2 700 000 inhabitants) for assessment of suitability for advanced therapies (heart transplant and ventricular assist devices). The unit was awarded double SEC-Excellence certification—as a specialized and an advanced HF unit—in 2017. Our hospital was also awarded SEC certification for excellence in ventricular assist device implantation in 2018.
The core HF unit team is formed by 5 cardiologists and 2 nurses. It offers the full spectrum of services for HF patients, including health education, treatment optimization, outpatient telemonitoring, ward care for patients admitted for decompensation, assessment of suitability for advanced therapies, and clinical follow-up after heart transplant or ventricular assist device implantation. To cover this array of services, the HF unit forms part of a broad multidisciplinary team comprising other cardiology units (imaging, hemodynamics, electrophysiology, intermediate care, family heart disease, congenital heart disease) and the hospital's heart surgery and intensive medicine departments. It also works closely with other departments and units (internal medicine, emergency medicine, home hospitalization, physiotherapy, social work, and mental health) and primary care centers.
Definition of variablesThe main outcome variable was all-cause mortality, which is the main survival indicator used in the SEC-Excellence program.5 The secondary outcome variable was the cumulative incidence of hospitalization for HF. We also studied changes in LVEF and New York Heart Association (NYHA) functional class from the start to the end of active clinical follow-up by the HF unit.
For the primary and secondary outcome variables, patients were followed up from baseline (first visit to the HF unit) to death or heart transplant, regardless of follow-up status. Data for patients who did not die or receive a heart transplant were censored on December 31, 2018. Survival data for patients discharged by the HF unit were obtained from the electronic medical record database held by the Galician Health System.
Predicted mortality at 1 and 3 years was calculated for all patients using the MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) risk score.6 This model predicts the probability of death using 13 clinical predictor variables derived from individual patient data for 39 372 patients from 30 studies, including 6 clinical trials. The patients represent a broad spectrum of HF patients, aiding the generalizability of the model.
The MAGGIC risk score has been externally validated in various populations of HF patients7–9 and has been found to have adequate calibration and discrimination and superior performance in this respect to other risk prediction models.10 The prognostic value of the MAGGIC risk score adds to that of natriuretic peptide levels.8,9
Statistical analysisQuantitative variables are presented as mean±SD or median (interquartile range [IQR]) depending on the normality of distribution. Categorical variables are reported as proportions. The Kaplan-Meier method was used to calculate the cumulative probability of death and hospitalization due to HF throughout follow-up; it was also used to build the corresponding survival curves.
Predicted 1- and 3-year mortality was calculated using the MAGGIC risk score. The discrimination power of the model in our population was analyzed using the c-statistic.
The difference between observed and predicted mortality was expressed as the observed/predicted ratio (O/P). The chi-square test was used to measure the statistical significance of this difference for the whole cohort, the quartiles of predicted risk, and several clinically relevant subgroups, defined by year of inclusion, age, sex, healthcare district of origin, LVEF, previous hospitalization for HF, and the presence of coronary artery disease.
All the analyses were performed in SPPS 20. Statistical significance was set at P<.5.
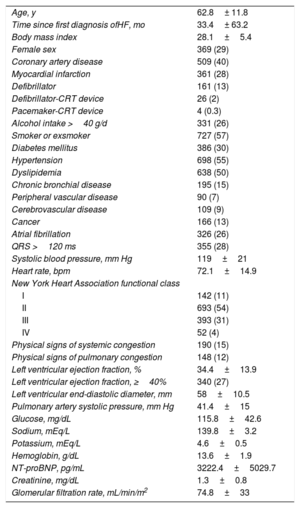
ResultsStudy populationBetween January 1, 2011 and December 31, 2017, 1280 patients with HF were seen for the first time at our HF unit. Of these, 648 (53.4%) had been hospitalized for HF at least once; 890 (69.5%) belonged to the healthcare district covered by our hospital and 390 (30.5%) were from other areas of Galicia. The clinical characteristics of the population are summarized in table 1.
Clinical characteristics of patients included in the study
| Age, y | 62.8± 11.8 |
| Time since first diagnosis ofHF, mo | 33.4± 63.2 |
| Body mass index | 28.1±5.4 |
| Female sex | 369 (29) |
| Coronary artery disease | 509 (40) |
| Myocardial infarction | 361 (28) |
| Defibrillator | 161 (13) |
| Defibrillator-CRT device | 26 (2) |
| Pacemaker-CRT device | 4 (0.3) |
| Alcohol intake >40 g/d | 331 (26) |
| Smoker or exsmoker | 727 (57) |
| Diabetes mellitus | 386 (30) |
| Hypertension | 698 (55) |
| Dyslipidemia | 638 (50) |
| Chronic bronchial disease | 195 (15) |
| Peripheral vascular disease | 90 (7) |
| Cerebrovascular disease | 109 (9) |
| Cancer | 166 (13) |
| Atrial fibrillation | 326 (26) |
| QRS >120 ms | 355 (28) |
| Systolic blood pressure, mm Hg | 119±21 |
| Heart rate, bpm | 72.1±14.9 |
| New York Heart Association functional class | |
| I | 142 (11) |
| II | 693 (54) |
| III | 393 (31) |
| IV | 52 (4) |
| Physical signs of systemic congestion | 190 (15) |
| Physical signs of pulmonary congestion | 148 (12) |
| Left ventricular ejection fraction, % | 34.4±13.9 |
| Left ventricular ejection fraction, ≥40% | 340 (27) |
| Left ventricular end-diastolic diameter, mm | 58±10.5 |
| Pulmonary artery systolic pressure, mm Hg | 41.4±15 |
| Glucose, mg/dL | 115.8±42.6 |
| Sodium, mEq/L | 139.8±3.2 |
| Potassium, mEq/L | 4.6±0.5 |
| Hemoglobin, g/dL | 13.6±1.9 |
| NT-proBNP, pg/mL | 3222.4±5029.7 |
| Creatinine, mg/dL | 1.3±0.8 |
| Glomerular filtration rate, mL/min/m2 | 74.8±33 |
CRT, cardiac resynchronization therapy; HF, heart failure; NT-proBNP, N-terminal fragment of brain natriuretic peptide.*
Data reported as mean ± standard deviation or number (%) of patients.
The patients were treated and monitored by the HF unit for a median of 621±863 days (range, 190-1053 days). In total, 427 patients (33.4%) were still under active follow-up on December 31, 2018. Of the 853 patients no longer under follow-up (66.6%), 598 (46.7%) had been discharged by the unit, 170 (13.3%) had died, and 85 (6.6%) had been referred for advanced therapy with a heart transplant or ventricular assist device.
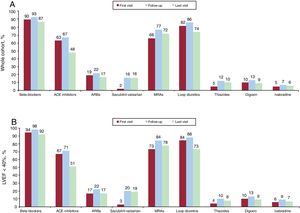
Pharmacological treatmentPrescription rates for the different classes of drugs at the first and last visits and during follow-up are shown in figure 1. Overall prescription rates for the cohort were 93% for beta-blockers, 67% for angiotensin-converting enzyme (ACE) inhibitors, 22% for angiotensin II receptor blockers (ARBs), 73% for mineralocorticoid receptor antagonists, and 16% for sacubitril-valsartan. The respective rates for the 940 patients with an LVEF <40% were 98%, 71%, 22%, 84%, and 22%. The proportion of patients with an LVEF <40% on sacubitril-valsartan still being monitored by the unit on December 31, 2018 was 43.2% (n=338).
Prescription rates by drug class at baseline, during follow-up, and at the end of follow-up for patients seen at the heart failure unit. A: Whole cohort. B: Patients with LVEF <40%. ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blockers; LVEF, left ventricular ejection fraction; MRAs, mineralocorticoid receptor antagonists.
The percentage of patients with an LVEF <40% on the target dose of beta-blockers increased from 18% at baseline to 29.6% at the last follow-up visit (P<.001). During the same period, there was also a significant increase in the proportion of patients with an LVEF <40% on the target dose of ACE inhibitors/ARBs/sacubitril-valsartan (11.3% vs 24.6%, P<.001). Finally, the proportion of patients with an LVEF <40% prescribed 50% or more of the target dose increased from 50.9% to 58.1% for beta-blockers (P<.001) and from 38.2% to 47.1% for ACE inhibitors/ARBs/sacubitril-valsartan (P<.001).
Nonpharmacological treatmentWhen seen for the first time at the HF unit, 161 patients (12.6%) had a defibrillator, 26 (2%) had a defibrillator-cardiac resynchronization therapy (CRT) device, and 4 (0.3%) had a pacemaker-CRT device. During follow-up, an additional 117 defibrillators, 91 defibrillator-CRT devices, and 10 pacemaker-CRT devices were implanted.
At the last follow-up visit, 211 (26.3%) of the 800 patients with a baseline LVEF <35% had a defibrillator, 78 (9.8%) had a defibrillator-CRT device, and 10 (1.3%) had a pacemaker-CRT device.
In addition, 6 patients (0.5%) underwent percutaneous mitral valve repair, 6 (0.5%) were fitted with a permanent ventricular assist device, and 81 (6.3%) underwent heart transplant.
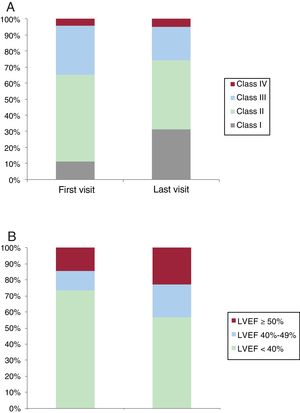
NYHA class and ejection fractionThe distribution of NYHA class changed significantly from baseline to the last visit (P<.001), with an increase in patients in NYHA class I (31.1% vs 11.1) and a decrease in patients in NYHA class II (42.9% vs 54.1) and III (20.9% vs 30.7%) (figure 2A). An improvement of at least 1 NYHA class was observed in 484 patients (37.8%).
There was also a significant improvement in LVEF from the first to the last visit (34.3%±13.9% vs 39.2±14.5%, P<.001). Of the 940 patients with an LVEF <40% at baseline, 239 (25.4%) had an LVEF ≥40% at the last visit. Just 25 patients (7.4%) experienced a reduction from an LVEF ≥40% to an LVEF <40% between the first and last visit. The distribution of LVEF values at baseline and the end of follow-up is shown in figure 2B.
OutcomesThe median survival follow-up time was 1238 days (IQR, 588-1888 days). During this period, 283 patients died (22.1%); 113 of these were no longer under follow-up at the unit, but they were nonetheless included in the survival analysis.
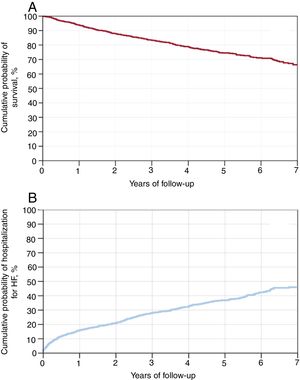
The estimated probability of survival according to the Kaplan-Meier method calculations was 99.7% for 30 days, 93.8% for 1 year, 83.3% for 3 years, and 74.5% for 5 years (figure 3A).
Overall, 413 patients (32.3%) were hospitalized for HF during the survival follow-up period. The cumulative probability of hospitalization due to HF was 4.2% for 30 days, 16.0% for 1 year, 28.3% for 3 years, and 36.8% for 5 years (figure 3B).
MAGGIC risk scoreThe median MAGGIC risk score was 19 [range, 13-24]. The score categories for the quartiles of predicted risk were 0-13 for the first quartile, 14-18 for the second quartile, 19-23 for the third quartile, and ≥24 for the fourth quartile. The distribution of scores is shown in figure 1 of the supplementary data.
The discrimination of the MAGGIC risk score (c-statistic) was 0.71 (95%CI, 0.65-0.77; P<.001) for 1-year mortality and 0.76 (95%CI, 0.72-0.81; P<.001) for 3-year mortality. The receiver operating characteristic curves are shown in figure 2 and figure 3 of the supplementary data.
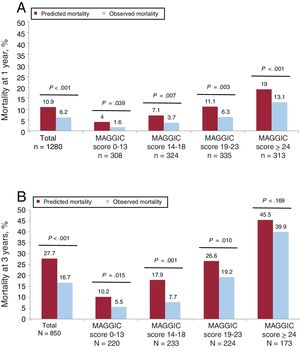
Predicted and observed mortalityThe comparative values for observed mortality and mortality predicted using the MAGGIC risk score for the full cohort and the 4 quartiles at 1 and 3 years are shown in figure 4A and figure 4B.
Observed mortality at 1 year was lower than predicted mortality in all cases, with an O/P of 0.57 for the whole cohort, 0.40 for the first quartile, 0.52 for the second quartile, 0.57 for the third quartile, and 0.69 for the fourth quartile. All the differences were statistically significant (P<.05).
Observed 3-year mortality was also significantly lower (P <.05) than predicted mortality for the whole cohort (O/P=0.60) and for the first (O/P=0.54), second (O/P=0.43), and third (O/P=0.43) quartiles. The difference for the fourth quartile was nonsignificant, with an O/P of 0.88 (P=.169).
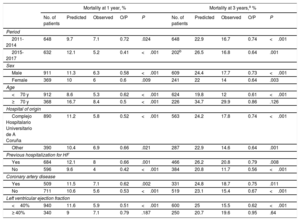
Clinical subgroupsThe results comparing observed and predicted mortality at 1 and 3 years are shown by subgroup (year of inclusion, sex, age, health care district of origin, previous hospitalization for HF, presence of coronary artery disease, and LVEF) in table 2.
Comparison of observed and predicted mortality at 1 and 3 years in different clinical subgroups
| Mortality at 1 year, % | Mortality at 3 years,a % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Predicted | Observed | O/P | P | No. of patients | Predicted | Observed | O/P | P | |
| Period | ||||||||||
| 2011-2014 | 648 | 9.7 | 7.1 | 0.72 | .024 | 648 | 22.9 | 16.7 | 0.74 | <.001 |
| 2015-2017 | 632 | 12.1 | 5.2 | 0.41 | <.001 | 202b | 26.5 | 16.8 | 0.64 | .001 |
| Sex | ||||||||||
| Male | 911 | 11.3 | 6.3 | 0.58 | <.001 | 609 | 24.4 | 17.7 | 0.73 | <.001 |
| Female | 369 | 10 | 6 | 0.6 | .009 | 241 | 22 | 14 | 0.64 | .003 |
| Age | ||||||||||
| <70 y | 912 | 8.6 | 5.3 | 0.62 | <.001 | 624 | 19.8 | 12 | 0.61 | <.001 |
| ≥70 y | 368 | 16.7 | 8.4 | 0.5 | <.001 | 226 | 34.7 | 29.9 | 0.86 | .126 |
| Hospital of origin | ||||||||||
| Complejo Hospitalario Universitario de A Coruña | 890 | 11.2 | 5.8 | 0.52 | <.001 | 563 | 24.2 | 17.8 | 0.74 | <.001 |
| Other | 390 | 10.4 | 6.9 | 0.66 | .021 | 287 | 22.9 | 14.6 | 0.64 | .001 |
| Previous hospitalization for HF | ||||||||||
| Yes | 684 | 12.1 | 8 | 0.66 | .001 | 466 | 26.2 | 20.8 | 0.79 | .008 |
| No | 596 | 9.6 | 4 | 0.42 | <.001 | 384 | 20.8 | 11.7 | 0.56 | <.001 |
| Coronary artery disease | ||||||||||
| Yes | 509 | 11.5 | 7.1 | 0.62 | .002 | 331 | 24.8 | 18.7 | 0.75 | .011 |
| No | 711 | 10.6 | 5.6 | 0.53 | <.001 | 519 | 23.1 | 15.4 | 0.67 | <.001 |
| Left ventricular ejection fraction | ||||||||||
| <40% | 940 | 11.6 | 5.9 | 0.51 | <.001 | 600 | 25 | 15.5 | 0.62 | <.001 |
| ≥ 40% | 340 | 9 | 7.1 | 0.79 | .187 | 250 | 20.7 | 19.6 | 0.95 | .64 |
HF, heart failure; O/P, ratio between observed and predicted mortality.
Observed 1-year mortality was significantly lower than predicted mortality for all subgroups except patients with an LEVF ≥40% (O/P=0.79, P=.187).
Observed mortality at 3 years was significantly lower than predicted mortality for all subgroups except patients aged 70 years or older (O/P=0.86, P=.126) and patients with an LVEF ≥40% (O/P=0.95, P=.640).
DiscussionWe have described outcomes for HF patients treated at a specialized, advanced care unit recently awarded SEC-Excellence certification. Our survival analysis of 1280 consecutive outpatients treated at the unit between 2011 and 2017 showed that actual (observed) mortality at 1 and 3 years was significantly lower than mortality predicted using the MAGGIC risk score. This underprediction was observed in different clinical subgroups, underlining the consistency of our findings.
An initial question regarding the design of our study concerns the suitability of using mortality predicted using the MAGGIC risk score as a comparator in a real-life treatment intervention. We believe that the choice of methodology, used previously,11 is justified by the difficulty of recruiting a control group of patients treated outside the HF unit. In addition, a randomized controlled trial design would raise serious ethical concerns, as it has already been shown that HF units reduce both morbidity and mortality.4 In addition, this evidence has already been incorporated into society guidelines.1,5 An observational study, in turn, would be clearly prone to selection bias, probably resulting in highly variable clinical profiles that would be difficult to correct for statistically.
Several studies have demonstrated the validity of the MAGGIC risk score for predicting mortality in outpatients with HF. Sartipy et al.,7 on analyzing its performance in 51 043 patients from a Swedish HF registry, observed good discrimination for 3-year mortality (c-statistic=0.741), albeit with slight underprediction (approximately −8%). Canepa et al.,10 in turn, studied the performance the MAGGIC risk score in 6161 HF patients seen between 2011 and 2013 from a European registry including data from several Spanish hospitals. They found good discrimination for 1-year mortality (c-statistic=0.743), with slight overprediction (+3%). More recently, the score was validated in Asian patients after hospitalization for HF.8,9
In our population, the MAGGIC risk score had good discrimination for both 1-year mortality (c-statistic=0.71) and 3-year mortality (c-statistic=0.76), and higher scores were directly proportional to increases in observed mortality. The model, however, had poor calibration due to systematic, statistically significant overprediction for 1-year (+75%) and 3-year (+66%) mortality for the group as a whole. The overprediction was observed in all the risk quartiles, except for 3-year mortality in the fourth quartile.
The magnitude of the difference between predicted and observed mortality in our population is remarkable. We cannot fully exclude the possibility that the discrepancy was due, at least in part, to the patients’ clinical characteristics. Some selection bias in patient referral to our unit appears to have occurred, as priority seems to have been given to middle-aged patients with predominantly systolic dysfunction without excessive comorbidity. Notwithstanding, we believe that this bias will have had a limited impact on results, as the main clinical variables that would have been affected are part of the MAGGIC model.6
The most likely explanation for the discrepancy between observed and predicted mortality are differences in how the patients in our cohort (seen at a contemporary HF unit) were treated and monitored compared with those whose data were used to create and externally validate the MAGGIC risk score.
In our series, a high proportion of patients were taking disease-modifying drugs, such as ACE inhibitors or ARBs, beta-blockers, and mineralocorticoid receptor antagonists. There was also a significant increase in the prescription of sacubitril-valsartan in the later years of the study. Adherence to prescribing recommendations in clinical guidelines has been shown to have a positive impact on prognosis,12 and improving this adherence, alongside treatment adherence among patients, is a key goal of HF units. These units are also an ideal setting for implementing nonpharmacological interventions with clinical benefits for HF patients, such as educational interventions in the areas of healthy living, self-care and identification of warning signs, correction of adverse psychosocial factors, early detection and treatment of nutritional deficiencies,13 and physical exercise.14 Nursing staff, working closely with colleagues from other departments and units (eg, mental health, nutrition, and physiotherapy) play a key role in these interventions.
Another goal of HF units with potential prognostic implications is to guarantee access to prophylactic treatment with CRT and/or defibrillation devices in suitable candidates.1 In our series, almost 40% of HF patients with a baseline LVEF <35% had a heart device during follow-up; similar rates have been observed in other registries of HF patients treated by cardiologists.15
Finally, HF units play an important role in identifying candidates for advanced therapies such as heart transplant and ventricular assist device implantation.16 Although quantitatively speaking these treatments have only a marginal impact on population health (as reflected by the low proportion of patients in our series who underwent these procedures [<7%]), they can have a dramatic impact on individual morbidity and mortality.
Actual mortality was lower than predicted mortality in all the subgroups of patients in our series, except in patients older than 70 years and patients with an LVEF >40%. Although patients with HF are frequently prescribed similar pharmacological treatments, regardless of their LVEF, no clear survival benefits have yet been demonstrated for patients with an LVEF >40%,17 and evidence is also lacking on the value of device implantation in this setting. Treatment optimization presents greater challenges in elderly patients, as they are more likely to have multiple comorbidities, preserved LEVF, and greater frailty and are also more prone to adverse drug-related effects.18 In addition, significant long-term survival benefits are less likely in this population because of chronobiological rhythms. It is therefore important to establish individualized treatment goals in elderly patients, prioritizing, in most cases, improved quality of life, adequate management of comorbid conditions, and reduced readmission rates.19
LimitationsThis study had several limitations. First, because it is an observational study conducted using information collected from a database, there is a risk of information bias. Second, it is a single-center study conducted in a highly specific care setting and our findings should therefore be generalized with caution.
As already discussed in detail, there are also limitations attached to the methodology used to compare observed and predicted mortality. Although the discrepancies between observed and predicted mortality in our series appear to be mostly attributable to differences in clinical treatment, we cannot be certain that our results were not influenced by differences in clinical variables that were not analyzed and that therefore could not be controlled for.
ConclusionsMortality at 1 and 3 years in a prospective cohort of patients treated and monitored at an HF unit within a cardiology department between 2011 and 2017 was significantly lower than that predicted using the MAGGIC risk score. This was true for the overall cohort and for all the clinically relevant subgroups analyzed except patients with an LVEF >40% and patients older than 70 years.
Our findings call into question the clinical usefulness of the MAGGIC risk score in contemporary care settings. Despite the methodological limitations already highlighted, they also point to the potential prognostic benefits of treating HF patients in specialized units.
FundingThe research group responsible for this study receives funding from the CIBERCV Consortium, which forms part of the Instituto de Salud Carlos III.
Conflicts of InterestNone declared.
- –
The Spanish Society of Cardiology (SEC) promotes the creation of dedicated units for the treatment of HF.
- –
The MAGGIC risk score is a model comprising 13 clinical variables that has been validated for predicting the risk of death in outpatients with HF.
- –
The findings of this study show that patients in a specialized HF unit who receive optimal treatment based on current guidelines have significantly lower mortality than that predicted using the MAGGIC risk score.
- –
Our findings support the role of specialized units in the clinical care of patients with HF.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2019.09.027