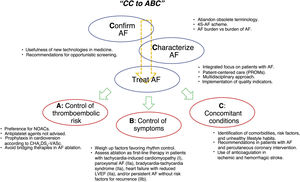
A new edition has been published of the guidelines for the management of atrial fibrillation (AF). The document contains a variety of pertinent changes. The guidelines delve into the organized approach to AF based on the CC (“confirm and characterize”) AF scheme and on integrated and multidisciplinary care. The authors propose that care be focused via the ABC pathway, which comprises the following 3 main areas of the approach to AF: a) anticoagulation/avoid stroke; b) better symptom management; and c) cardiovascular and comorbidity optimization. The guidelines stress that this line of action (figure 1) would directly reduce cardiovascular event risk and require a more active participation of various professionals and of patients themselves in therapeutic decision-making and their own care.1 In general, these are the messages underlying each of the sections of the new document, which we discuss here one by one.
DEFINITION, DIAGNOSIS, AND SCREENINGThe diagnosis of clinical AF continues to be consolidated around its electrocardiographic documentation but major new inclusions are the use of portable devices (smartphones and smartwatches) that calculate heart rhythm through analysis of the photoplethysmographic signal, direct recording of the electrocardiographic signal, or a combination of the 2 techniques. These approaches permit the diagnosis of subclinical AF, although the predictive capacity varies among techniques and devices. Their potential use is highly widespread but they are also a source of doubt because the subclinical AF episodes detected do not necessarily correspond to the risk levels already identified in patients with clinical AF. In addition, their specificity can be limited, which could unnecessarily increase health care demand. This is undoubtedly a controversial area, and parameters need to be established to enable the validation and regulation of the handling of the data recorded by these devices. Nonetheless, it is recognized that they may play a major role in the AF screening performed by patients themselves. The Apple Heart and Huawei Heart studies found positive predictive values of 34% and 87%, respectively, which highlight their usefulness but also the need for their confirmation by trained health care professionals.1 Nonetheless, conventional clinical methods for AF screening are strengthened (eg, pulse palpation, with various recent meta-analyses confirming its usefulness). Also recommended is opportunistic screening of patients older than 65 years (class I) and systematic screening of those older than 75 years or with high stroke risk (IIa), given that effectiveness increases with repeat screening.
EPIDEMIOLOGY AND CLINICAL PRESENTATIONNew data are highlighted that indicate an AF prevalence of between 2% and 4% in the general population and that maintain it as the most common sustained arrhythmia in adults. In addition, its prevalence is expected to double due to increased population longevity (confirming age as the most powerful risk factor), more intense screening, and the incorporation of subclinical AF into the clinical panorama. The already classic temporal classification of AF is maintained without changes from previous years (first detected, paroxysmal, persistent, long-standing persistent, and permanent) but the document explicitly recommends abandoning the terms lone AF, valvular vs nonvalvular AF, and chronic AF due to the confusion that they cause and their lack of clear pathophysiological evidence. Moreover, a change from this classification to the structured characterization of AF (IIa) is promoted through the novel 4S-AF scheme, which considers 4 key aspects with therapeutic and prognostic implications: stroke risk, symptom severity (stressing the value of the EHRA scale), severity of AF burden, and substrate. This scheme simplifies the evaluation at all health care levels, facilitating communication among health care professionals, decision-making, and the optimal treatment of patients, and should become the standard approach in our health care system. The authors explain the term AF burden as the time spent by patients with AF episodes (clinical or subclinical). An original aspect is that this term is differentiated from the burden or impact of the clinical manifestations of AF (“burden of AF”). How these 2 concepts are to be used in decision-making remains to be defined, although there is a need for a certain degree of patient-focused judgment.
INTEGRATED APPROACHThe guidelines recommend integrated and patient-individualized and -centered management (I). This holistic focus requires a coordinated interdisciplinary team (cardiology, primary care, nursing, and pharmacy), as well as the empowering of patients regarding education, lifestyle, and decision-making, which are key to successful management.2 The capacity, opportunity, and motivation of staff must be considered and it is important to prioritize a cohesive multidisciplinary focus on information dissemination and doubt resolution. One of the points highlighted by the guidelines is the clear communication between cardiology and primary care. A major development is the inclusion of nursing staff specializing in AF as a fundamental part of the team. Nurse-led AF clinics, recently implemented in Spain, help to improve patient education, risk factor control, and adherence to medication and healthy lifestyles.3 We are urged to use quality indicators, such as health-related quality of life or patient-reported outcome measures (PROMs), which, measured during follow-up, should boost the therapeutic strategy, the determination of outcomes, and care improvements (I). The objective is to balance outcomes and indicators to achieve a treatment intensity that is accepted by patients and provides them with the best quality of life and health.4 In addition, this approach would enable the evaluation of a structured treatment in line with the proposed ABC scheme (anticoagulation/avoid stroke; better symptom management; cardiovascular and comorbidity optimization).
ANTICOAGULATION AND STROKE PREVENTIONThe scales recommended for risk evaluation are CHA2DS2-VASc and HAS-BLED, which require consideration of the specific value of each variable. Hemorrhagic stroke increases the risk of new ischemic stroke and must be considered in the scale. History of hypertrophic cardiomyopathy must be noted and female sex is a modifier rather than a risk factor. The authors stress that biomarkers (troponin or natriuretic peptide elevation) can aid decision-making in doubtful cases with a single risk factor, as well as the importance of a dynamic assessment (periodic reassessment of low-risk individuals). Bleeding risk must be used to identify modifiable factors and to correct them, if possible, and never to establish the contraindication to anticoagulation, which is only considered in cases of active bleeding, thrombocytopenia (< 50 000 platelets), severe anemia under investigation, or recent intracranial hemorrhage.
Regarding the anticoagulant of choice, clear preference is given to nonvitamin K antagonist oral anticoagulants (NOACs) due to their better safety and efficacy profiles. This is a major aspect in Spain because the Therapeutic Positioning Report (IPT) uses a visa system to restrict the financing of NOACs in Spain to special situations. In addition, a time in therapeutic range (TTR) <60% to 65% (depending on methodology) is required to obtain financing, whereas the guidelines consider a TTR <70% independently of methodology. More than 50% of patients in Spain taking vitamin K antagonists (VKAs) have deficient control,5 which has led scientific societies and professionals to warn about the risks of the current IPT.6 They highlight the frequent inappropriate reduction in dosage, which reduces effectiveness but not complications. In addition, there are major regulatory differences among the autonomous communities of Spain that lead to a manifest inequity in access to NOACs as first-line therapy. Antiplatelet therapy is not recommended as prophylactic therapy for thromboembolic disease (III).
The guidelines have few indications regarding appendage closure (an absolute contraindication for oral anticoagulation [IIb]) and there is a notable absence of a clearly studied antithrombotic regimen for these patients. A recent document from the EHRA7 adds other indications (such as poor adherence or anticoagulant rejection and stroke despite appropriate anticoagulation) that are not considered in the new guidelines due to the absence of clinical trials or the lack of comparisons vs NOACs.
SYMPTOM CONTROLHeart rate controlThis section contains no major changes from the previous recommendations (heart rate <110 bpm unless stricter control is required due to symptoms, ventricular function deterioration, or need to ensure continuous biventricular pacing). Beta-blockers, together with verapamil and diltiazem, are first-line drugs that are a good alternative in patients with preserved left ventricular ejection fraction (LVEF). Their combination with digoxin is a second-line therapy after failure of optimal control. The outcomes of the RATE-AF study,8 presented at the same ESC 2020 conference as these guidelines, will probably affect this indication in future editions, given the apparent benefits of digoxin over beta-blockers for rate control therapy in elderly patients. Antiarrhythmic agent administration is not recommended if the aim is merely to achieve good heart rate control, although amiodarone can be useful in patients who are not candidates for nonpharmacological control and who need combination drug therapy. AV nodal ablation is recommended after drug therapy failure. The new forms of pacing are reported as an alternative. In patients with structural heart disease or ventricular dysfunction, resynchronization via biventricular pacing is a first-line treatment, with para-Hisian pacing considered a reasonable alternative.9
Rhythm controlThe objective of rhythm control is to improve symptoms and quality of life (I). The release of these guidelines coincides with the publication of the results of the EAST-AFNET 4 trial,10 which compared a rhythm control strategy based on cardioversion, antiarrhythmic drugs, and catheter ablation (20% at 2 years) vs routine clinical practice (largely rate control) in new-onset AF (less than 1 year). The rhythm control strategy was associated with a significant 21% reduction in the primary outcome (which included cardiovascular death), with no increase in adverse effects. The frequency of sinus rhythm maintenance at 2 years follow-up was significantly higher in the rhythm control group (82% vs 62%). Accordingly, and providing some further nuance to the guideline recommendations, this study provides data suggesting that the prognosis of patients with recently diagnosed AF is improved by early recovery and maintenance of sinus rhythm. One novel aspect is that a 48-hour “wait and watch” strategy (cardioversion only if the AF does not yield) in patients with recent AF (< 48hours) seems to be as effective as immediate conversion in maintaining sinus rhythm at 4 weeks (although it would require the appropriate infrastructure, such as short-stay units).1
The indication for ablation should be individualized. In general, the guidelines suggest catheter ablation11 after the failure of at least 1 antiarrhythmic agent (I). However, if the patient agrees, it can be the first-line treatment in patients with tachycardia-induced cardiomyopathy (I), paroxysmal AF (IIa), bradycardia-tachycardia syndrome (IIa), heart failure with reduced LVEF (IIa), and persistent AF without risk factors for recurrence (IIb). The recently reported results of the STOP AF First study12 support ablation as the first-line treatment of paroxysmal AF. This was the first randomized clinical trial to show higher efficacy of balloon cryoablation of the pulmonary veins vs antiarrhythmic drugs in the initial treatment of paroxysmal AF. The indication (I) is maintained for pulmonary vein isolation as first-line ablation, independently of the technique used (cryoablation or point-by-point radiofrequency ablation). The treatment of other ablation targets receives a lower recommendation (IIb), which is why it should be restricted to specific patients. A controversial aspect is the role of ablation in selected patients with AF and heart failure with reduced LVEF. Although the guidelines award ablation a IIa recommendation in this situation, the document does not provide practical information facilitating patient selection and omits the pertinent data derived from the CAMERA-MRI study,13 in which quantification of left ventricular fibrosis enabled identification of the patients who would most benefit from ablation.
The effectiveness of AF surgery in sinus rhythm maintenance is recognized, but doubts remain about its clinical benefit. The recommendations exist in an atmosphere of relative uncertainty, given that they include heterogeneous data. In addition, the data are largely derived from registries; moreover, there are differences among the studies in the type of patients included and the type of AF, although this treatment is generally reserved for long-standing persistent AF. Another heterogeneous variable concerns the technique applied to treat the substrate, both in the design of the lines and in the energy and tools used. The most frequent indication is concomitant surgical ablation in patients undergoing another surgery (IIa). When they are not associated with another surgery, thoracoscopic procedures are largely reserved for patients who have failed percutaneous AF ablation (IIa), although the downside is a higher number of complications. In long-standing persistent AF, the recent CASA-AF study,14 which randomized participants to a percutaneous or thoracoscopic approach, failed to show any advantage of the surgical approach. Compared with purely percutaneous procedures, hybrid therapy offers higher rates not only of effectiveness, but also of complications. The section on long-term antiarrhythmic therapy shows no major changes. A new algorithm summarizes the choice of drugs and catheter ablation in the different clinical situations.
Anticoagulation in cardioversion/ablationThe document reports the impact of the incorporation of NOACs into clinical practice and promotes safety, effectiveness, and efficiency (eg, in the better control of preprocedural waiting times). An aspect that is addressed with special emphasis and diverse novelties is that of cardioversion-related thromboembolic risk. Maintaining a IIa indication for early cardioversion in patients not previously coagulated with an AF duration <48hours, the guidelines recommend individualization by clinical context. The authors reiterate that the available data indicate a good safety profile in patients with a definite AF duration <12hours and CHA2DS2-VASc scores of 0 in men or 1 in women. However, the evidence for other patient profiles, such as that of those with AF of between 12 and 48hours and higher CHA2DS2-VASc scores, is not as robust and their risk could be higher. These patients are candidates for a delayed cardioversion strategy, even if their AF duration is less than 48hours. Regardless, the guidelines strengthen the concept of prioritizing safety and recognize the role played by transesophageal echocardiography in different situations. In contrast to previous guidelines, in patients with CHA2DS2-VASc scores of 0 in men and 1 in women, oral anticoagulation is indicated for 4 weeks after cardioversion of an AF with a duration> 24hours (this was set at> 48hours in the previous guidelines). If the AF duration is “definitely” <24hours, the indication is now optional. Regarding AF ablation, the clear preferences are uninterrupted treatment schedules (vs bridging therapy) and NOACs. In this regard, few recommendations are listed, although their recommendation levels are high. Once again, long-term anticoagulation should be maintained based on the patients’ risk profile and not on intervention success.
RISK FACTORS, COMORBIDITIES, AND SPECIAL CONDITIONSImportant aspects of the integrated approach are the identification of comorbidities, risk factors, and unhealthy lifestyle habits. Adequate control of these factors helps to limit their progression and symptoms and reduce stroke risk, which clearly affects prognosis and improves quality of life and the rate of recurrence after ablation. Taken together, the promotion of heart-healthy habits and the appropriate treatment of the factors and conditions associated with arrhythmia has a class I recommendation. However, somewhat surprisingly, the recommendation has been downgraded for the optimized treatment of patients with obstructive apnea and regular exercise (I B). In this regard, there is a slight discrepancy from the guidelines on sports cardiology and exercise in patients with cardiovascular disease,15 published at the same time. In that document, physical activity is assigned a I A recommendation for preventing AF in patients with the condition. Although the wording of the specific recommendations of each guideline is different (that of AF jointly takes into account the special situation of high-intensity sports), this should not affect the level of evidence, which is largely based on the quality and type of studies available.
There are some changes to the antithrombotic treatment recommendations in patients with AF undergoing percutaneous coronary intervention. After an adequate consideration of ischemic and bleeding risks, the guideline recommendations are as follows: a) a NOAC plus clopidogrel should be administered for 12 months in acute coronary syndrome and for 6 months in chronic coronary syndrome; b) dual therapy with NOACs is preferred to VKAs; and c) triple therapy should be limited to the first week, with the possibility of prolonging it to 1 month when there is elevated risk of ischemia or of stent thrombosis (IIa). It is important to note that the levels of evidence regarding this recommendation are B and C. Notably, the guidelines do not consider the differences between acute and chronic coronary syndrome, despite the differing thrombotic risk of the 2 entities. In addition, at the time of individualized clinical decision-making, it must be remembered that only one of the trials evaluated the early withdrawal of aspirin, as well as the low power of the studies performed in the detection of thrombotic events. The guidelines discourage the use of prasugrel and ticagrelor. However, these drugs can be part of the dual therapy strategy (a single antiplatelet agent plus anticoagulation) in patients with moderate-high thrombotic risk according to the guidelines on non–ST-segment acute coronary syndrome,16 published simultaneously. This point of discrepancy between the 2 documents requires clarification. In AF patients treated with surgical revascularization, anticoagulation must be restarted as soon as the bleeding is controlled, possibly in combination with clopidogrel and avoiding triple therapy. In patients with AF and stroke, previous use of anticoagulation contraindicates thrombolysis due to bleeding risk, except when the last NOAC dose was administered more than 48hours before and renal function is normal. In the remaining cases, patients should undergo endovascular treatment. Some patients treated with dabigatran could receive fibrinolysis if the antidote idarucizumab is available. The authors stress that anticoagulation initiation within 48hours (after ischemic stroke) is related to hemorrhagic transformation and higher mortality and is not recommended (III). In addition, NOAC administration in secondary prevention is recommended over VKAs (I A) in stroke patients.
There are few developments in cryptogenic and embolic stroke with undetermined source. The current evidence does not support routine oral anticoagulant (OAC) use and opts for monitoring during the first 72hours (I). Additional long-term monitoring can be considered (class IIa) to increase detection possibility. Some of the unsolved questions are the detection time required for OAC initiation or its cost-benefit. There is no evidence supporting screening with mobile devices, as described previously. In the case of intracranial hemorrhage, it seems reasonable to delay the reinitiation of anticoagulation beyond the acute phase (4 weeks) and, in patients with very high risk of recurrence, evaluate appendage closure (IIb), with due consideration of the existing limitations regarding the necessary antithrombotic therapeutic regimen.
NOACs are contraindicated in patients with valvular heart disease with moderate-severe mitral stenosis or a mechanical prosthesis. It is important to remember that the situation is different for the remaining valvular heart diseases, including stenosis/aortic regurgitation, mitral regurgitation, and patients with a bioprosthesis or valve repair, because there is no evidence supporting alteration of the anticoagulant of choice. Elderly patients obtain more net clinical benefit from anticoagulant therapy, although a high percentage does not unfortunately receive anticoagulation but antiplatelet agents. In adults with congenital heart diseases and AF, the authors note the high embolic risk of the complex forms (IIa for anticoagulation). In other types, the recommendation is maintained in patients with embolic risk factors. There is also a IIa recommendation for the surgical treatment of AF in patients undergoing other interventions, if they have symptomatic episodes. In pregnancy, rhythm control is preferred, with anticoagulant therapy determined by CHA2DS2-VASc score and the drug therapy adapted according to trimester of pregnancy, as specified in the specific guidelines, and remembering that NOACs are contraindicated. Professional athletes have a 5-fold increased risk of AF.
Anticoagulation must be guided by the CHA2DS2-VASc score and the routine choice is rhythm control using ablation (in patients with intolerance to the drugs in question or those not recommended to exercise after taking flecainide). Regarding postoperative AF, the evidence supporting long-term anticoagulation is not based on clinical trial data but on observational data. Nonetheless, the guidelines recommend chronic anticoagulation in patients with elevated risk after noncardiac surgery (IIa) and with less weight after cardiac surgery (IIb). Two ongoing randomized studies will provide useful data. An aspect not addressed in the current guidelines is the relationship between AF and patients with solid tumors, of interest due to the elevated prevalence of AF at diagnosis of neoplastic disease and the increased arrhythmic risk associated with different antineoplastic therapies (eg, directed, chemotherapy, immunotherapy).
ATRIAL FIBRILLATION IN WOMENThe guidelines analyze the particular situation of AF in women, who are classically underrepresented in clinical trials. As a general rule, AF is diagnosed in women at an older age, which may explain the modifying role of female sex in terms of its association with a higher incidence of stroke and of more severe stroke; in addition, the higher prevalence of hypertension, vascular disease, and heart failure with preserved LVEF in women explain the circumstantial, rather than causal, relationship between female sex and AF. The guidelines advise against differentiation by sex in terms of AF-related diagnostic and therapeutic options.
IMPLEMENTATION AND QUALITY INDICATORSOne of the aspects repeatedly emphasized throughout the document is that the ultimate aim of the guidelines is to improve patient-centered outcomes. Adherence to guideline-recommended treatments is associated with better health outcomes. However, multiple international registries of AF show that there is room for improvement and significant geographical variability. The new guidelines recommend the application of quality indicators to identify opportunities for improvement (IIa) and advise the use of the standardized measures reported in a simultaneously published document dedicated to quality indicators,4 the result of a collaboration among international arrhythmia and patient associations. This is the first time that guidelines have endeavored to simultaneously define quality indicators to quantify their implementation and their impact on patient-centered AF outcomes. The use of the recommended indicators will have to be validated (implementation) to confirm their usefulness in routine clinical practice, an approach promoted by the Spanish Society of Cardiology within the SEC-Excelente framework and the AF process.
ATRIAL HIGH-RATE EPISODES/SUBCLINICAL ATRIAL FIBRILLATIONThe detection of high-rate episodes or subclinical AF is an area of growing interest. The relevant line of thought can be summarized as follows: a) they are mainly detected in patients with therapeutic devices, with an incidence ostensibly higher than that of the general population; b) they increase the risk of clinical AF and thromboembolic events according to duration (irrelevant in brief implantations), although their effect is lower than that of clinical AF; c) they exhibit temporal variability and a temporal dissociation from thromboembolic events, which indicates their role as a marker more than as a causative agent; and d) the indication for anticoagulation is being studied in randomized trials (NOAH-AFNET 6 and ARTESiA); it can currently be considered in the presence of prolonged episodes (> 24hours) in patients with an elevated CHA2DS2-VASc score. The therapeutic decision should be based on a complete evaluation of the clinical situation, thromboembolic risk, and arrhythmia burden.
CONCLUSIONSThese new guidelines on AF management stand out for their largely clinical interpretation key, addressing aspects that provide value in the care of patients with AF and promoting an integrated focus that stresses the patients’ perspective. It is worth noting the alignment observed regarding the working framework proposed by the SEC-Excelente processes that addresses AF and clarifies the benefits of a structured and quantifiable approach based on appropriate quality indicators. Their practical application in Spain appears, evidently, plausible and necessary. Within a value-focused strategy, NOACs acquire a pertinent role and are a pending aspect in our health care system that will have to be assessed within the appropriate framework. Finally, and with reference to the comments made in previous years,17 AF continues to be a field with multiple uncertainties. Although the number of recommendations with level of evidence A has increased from 15% to 17%, the scope for research into AF-related aspects and the care of such patients continues to be extensive.
CONFLICTS OF INTERESTThe following authors report relationships not related to the current work: E. Arbelo, honoraria for conferences from Biosense Webster; F. Arribas, honoraria for consulting, advice, or conferences from Daiichi Sankyo, Impulse Dynamics, Medtronic, Boston Scientific, Bayer, Bristol Myers Squibb, and Abbott and institutional grants or aid from Novartis, Janssen-Cilag, Medtronic, Boston Scientific, Daiichi Sankyo, Bayer, Biosensors, and Edwards Lifesciences; F. Atienza, patents licensed to the Polytechnic University of Valencia and is a member of the advisory committees of Medtronic and MicroPort; V. Barrios, honoraria from Boehringer Ingelheim, BMS/Pfizer, and Daiichi Sankyo; D. Calvo, honoraria for consulting, advice, or conferences from Daiichi Sankyo, Bayer, Pfizer, Boehringer Ingelheim, and Novartis and research contracts with Biosense Webster and Medtronic; J. Cosin-Sales, research grants from BMS/Pfizer and honoraria for consulting and conferences from Bayer, BMS/Pfizer, Daiichi-Sankyo, Boehringer Ingelheim, and Cardiome; J.M. Gámez, honoraria for conferences from Daiichi-Sankyo and Pfizer; F. Marín, honoraria from Boehringer Ingelheim and AstraZeneca and nonpecuniary assistance from Esteve. The other authors have no conflicts of interest to declare.
SEC Working Group for the 2020 ESC guidelines for the management of atrial fibrillation: David Calvo (coordinator), Elena Arbelo (coordinator), Fernando Arribas, Juan Cosín, José María Gámez, Javier Jiménez Candil, Miriam Juárez, Francisco Marín, Silvia Pérez Ortega, and Pablo Jorge Pérez.
Expert reviewers for the 2020 ESC guidelines for the management of atrial fibrillation: Concepción Alonso, Albert Ariza, Felipe Atienza, Vivencio Barrios, Begoña Benito, Vicente Bertomeu, Carlos Escobar, Esteban López de Sá, Ana Martin, Roberto Martín Asenjo, Marta Pachón, and Marta Pombo.
SEC Guidelines Committee: Pablo Avanzas, Gemma Berga Congost, Araceli Boraita, Héctor Bueno, David Calvo, Raquel Campuzano, Victoria Delgado, Laura Dos, Ignacio Ferreira-González, Juan José Gómez Doblas, Domingo Pascual Figal, Antonia Sambola Ayala, Ana Viana Tejedor, José Luis Ferreiro (copresident), and Fernando Alfonso (copresident).
.