The most important aspect of these guidelines is the adoption of a “new” classification of ischemic heart disease, categorizing the entity as either acute coronary syndromes (ACSs) or chronic coronary syndromes (CCSs).1 The purpose of this change is not only to highlight the dynamic nature of the ischemic heart disease process, but also to facilitate the categorization of the various possible clinical presentations. This has also led to a change in the name of the guidelines: they now refer to the diagnosis and management of “CCSs”, in contrast to “stable coronary artery disease” in 2013.2 Specifically, CCSs encompass 6 situations with different risks for adverse cardiovascular events (table 1); this risk can change over time and, of course, decrease with revascularization and the appropriate use of secondary prevention measures. In addition, the guidelines consider the pretest probability of coronary artery disease (CAD) according to age, sex, and type of symptoms.
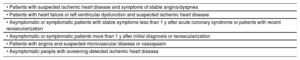
Clinical situations included in the concept of chronic coronary syndrome
| • Patients with suspected ischemic heart disease and symptoms of stable angina/dyspnea |
| • Patients with heart failure or left ventricular dysfunction and suspected ischemic heart disease |
| • Asymptomatic or symptomatic patients with stable symptoms less than 1 y after acute coronary syndrome or patients with recent revascularization |
| • Asymptomatic or symptomatic patients more than 1 y after initial diagnosis or revascularization |
| • Patients with angina and suspected microvascular disease or vasospasm |
| • Asymptomatic people with screening-detected ischemic heart disease |
A 6-step diagnostic approach is proposed for patients with angina and suspected CAD (Figure 2 of the guidelines1).
Step 1: signs and symptomsRegarding symptoms, the guidelines reiterate the typical, atypical, and nonanginal chest pain angina classifications. According to a recent study,3 typical angina is an uncommon presentation. Unstable angina should be treated as an ACS, except for low-risk angina without recurrence, which is characterized by no signs of heart failure (HF), ECG changes, or troponin elevation and which should be managed via a noninvasive diagnostic strategy.
Step 2: comorbidities and other causes of symptomsThe guidelines stress the importance of assessing body mass index, searching for noncardiac vascular disease, arrhythmias, and valvular or hypertrophic heart disease, and evaluating quality of life and comorbidities such as anemia, thyroid dysfunction, diabetes, and kidney disease before ordering other tests. The role of resting ECG is unchanged: it should be performed in all patients with low CAD suspicion.
Step 3: basic testingThe main novelties regarding the assessment of patients with angina or dyspnea and suspected CAD focus on assessing the pretest probability of CAD and selecting the diagnostic techniques. The probability of obstructive CAD depends on the prevalence of the disease in the studied population. The previous guidelines adhered to the classification developed by the CAD Consortium (an updated version of the Diamond and Forrester classification) to determine the pretest probability of obstructive CAD.4 The results of a meta-analysis that included 3 contemporary populations showed that the pretest probability of obstructive CAD was one third of that estimated with the model used in the previous guidelines.5
Step 4: assess pretest probability and clinical likelihood of coronary artery diseaseThe guidelines1 provide Table 5 to calculate the pretest probability of CAD. This approach will probably reduce the number of diagnostic tests ordered, both noninvasive and invasive; in addition, dyspnea is noted as one of the symptoms that should be considered. Patients with a pretest probability <15% can be discharged without undergoing any diagnostic test because the pretest probability of infarction is <1%. Nonetheless, the table minimizes the risk of CAD in women, given that a 50- to 59-year-old woman (most of whom would be postmenopausal) with typical chest pain has a pretest probability of 13%. However, a man of the same age with atypical chest pain has a pretest probability of 17%. Thus, according to the recommendations of these guidelines, many women should be discharged without undergoing any additional tests, almost guaranteeing the underdiagnosis of CAD.6,7 In addition, this pretest probability can be modified according to age, sex, and type of symptoms (Figure 3 of the guidelines1). However, how this risk is modified has not been quantified.
Step 5: select appropriate testingThe main changes in the recommendations include the class I indication for computed tomography (CT) coronary angiography as the initial test to diagnose obstructive CAD, as well as other stress imaging tests (echocardiography, cardiac magnetic resonance imaging, positron emission tomography, and single-photon emission computed tomography); exercise ECG is now level IIb and is now recommended only when there is no access to imaging techniques. The imaging technique selected will depend on availability and experience in the diagnostic center and on patient characteristics. However, the radiation inherent to some imaging techniques (eg, SPECT) is not considered nor is the need to prioritize exercise echocardiography over these modalities.
A noninvasive stress imaging test is recommended when there is a high clinical probability of CAD, previous revascularization, or need to assess myocardial viability. In addition, invasive coronary angiography is considered reasonable—without any prior diagnostic test—in patients with a high pretest probability of obstructive CAD or with left ventricular dysfunction. In this setting, the need for revascularization will be determined by the invasive confirmation of hemodynamically significant coronary stenosis. An anatomical (CT coronary angiography) or functional (ischemia detection) imaging test is required in patients with an intermediate pretest probability of CAD.
In patients with irregular cardiac rhythm and high suspicion of coronary calcification, CT coronary angiography may have little diagnostic accuracy, whereas the risks of imaging techniques that involve radiation or nephrotoxic contrast agents should be judged based on their diagnostic benefits when indicated for young patients or those with kidney disease.
No major changes are made from previous guidelines in terms of invasive tests. In summary, invasive coronary angiography is recommended under the following circumstances: when a noninvasive diagnostic test cannot be performed or is inconclusive; in people from certain professions due to regulatory issues; when noninvasive test results show a high risk of cardiovascular events; in patients with high probability of CAD and with typical angina who have low levels of exercise or do not improve with medical therapy; and in patients with ventricular dysfunction probably related to CAD. As in previous guidelines, the current document recommends systematic invasive evaluation of the fractional flow reserve (FFR) in intermediate lesions (50%-90%) or in patients with multivessel disease. A novelty of these guidelines is the inclusion of new indices that do not require hyperemia.
Step 6: assessment of event riskAs before, event risk should be evaluated in all patients with suspected CAD or a new diagnosis. However, a novel change is the recommendation to evaluate ejection fraction using echocardiography before invasive coronary angiography, due to its major impact on therapeutic decisions. In addition, invasive coronary angiography and additional assessment using FFR are considered to be hugely valuable for the risk stratification of certain selected patients.
Lifestyle interventionsThe guidelines stress the importance of lifestyle interventions involving a mixed and multidisciplinary approach based on heart-healthy lifestyle habits and optimal drug therapy, as well as cognitive behavioral interventions and cardiac rehabilitation programs. The authors highlight the ability of nurse-led cardiovascular risk control programs to achieve cardiovascular prevention targets by increasing therapeutic adherence.
Regarding smoking, drug therapies involving bupropion, varenicline, or nicotine substitutes are considered safe and effective. For the first time, electronic cigarettes are mentioned as an alternative way to achieve smoking cessation. Nonetheless, the guidelines note their detrimental effects, caused by the vaporization of harmful substances such as carbonyls.
Based on a recent study,8 the guidelines state that abstinence is the level of alcohol consumption that minimizes health risks. However, if alcohol is to be consumed, an intake of <15g/d (or 100g/wk) is recommended. In contrast to previous guidelines, the recommended alcohol limits do not differ according to sex. As for other drinks, the guidelines mention that sugar-sweetened soft drinks should be avoided due to their negative impact on atherosclerosis. The Mediterranean diet is still recommended. Physical exercise recommendations have been increased to 30 to 60min/d at least 5 days a week. In addition to aerobic activity, resistance exercise is also recommended for the first time to improve insulin sensitivity and blood pressure and lipid control. The guidelines stress that the risk of acute myocardial infarction (AMI) or sudden cardiac death during sexual intercourse is very low and is even lower if those involved take regular physical activity.
As a novel aspect, the authors highlight the importance of cardiac rehabilitation in patients with CCSs (I A indication) and not only in those who have had an ACS. This aspect might be difficult to implement in Spain due to the insufficient availability of cardiac rehabilitation units. Finally, the authors refer to environmental factors, noting that exposure to air pollution increases cardiovascular risk and mortality. In this regard, the guidelines recommend air purifiers with particle filters and face masks.
Drug therapyThe guidelines continue to recommend beta-blockers and calcium antagonists as first-line antianginal therapy, with a target heart rate of 55 to 60 bpm. Although the authors recognize the class effects of all cardioselective beta-blockers, in the case of calcium antagonists, the guidelines mention amlodipine and nifedipine as dihydropyridines and verapamil and diltiazem as nondihydropyridines. Nitrates remain a second-line treatment, with a higher recommendation class than that of other antianginal agents (ivabradine, ranolazine, trimetazidine, and nicorandil). The absence of an improvement in cardiovascular mortality and reinfarction in ivabradine studies in patients with preserved systolic function,9 as well as with ranolazine and nicorandil, might be at least partly why these 3 drugs have lost the class IIa indication of previous guidelines and are now a class IIb indication as first-line antianginal treatments (same level of recommendation as trimetazidine); they are still indicated as second-line treatments due to their efficacy in treating anginal symptoms (class IIa).
As a novelty, the guidelines propose a therapeutic algorithm with 3 levels (4 in patients with a heart rate <50 bpm) according to different clinical situations (heart rate> 90 bpm, heart rate <50 bpm, hypotension, and ventricular dysfunction), selecting the most appropriate antianginal therapy for each situation. Likewise, a reassessment of antianginal effectiveness is advised at 2 to 4 weeks.
The guidelines recommend beta-blocker therapy with a I A indication in patients with systolic dysfunction (left ventricular ejection fraction [LVEF] <40%) and with a IIa B indication in patients with preserved LVEF and history of ST-segment elevation AMI. This clinical strategy is controversial, particularly in patients undergoing coronary revascularization, because the results of various retrospective studies and meta-analyses disagree about its clinical benefit.10
Most of the section on event prevention is devoted to antithrombotic therapy and its different options, which depend on patients’ clinical situation, and a careful and individualized assessment of ischemic and bleeding risks is always recommended to determine the treatment strategy. Regarding the duration of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI), 6-month DAPT is still generally recommended. The duration of this therapy can be shortened or lengthened depending on patients’ ischemic and bleeding risks; clopidogrel is the P2Y12 inhibitor of choice, but prasugrel or ticagrelor may be considered in high-risk situations.
A notable recommendation related to long-term secondary prevention is to add a second antithrombotic drug to aspirin in patients with highly (IIa) or moderately (IIb) increased risk of ischemic events and low bleeding risk. In addition to P2Y12 inhibitors (clopidogrel, prasugrel, or ticagrelor at a dose of 60mg/12h), the various options for the second drug include low-dose rivaroxaban (2.5mg/12h). This drug is recommended for the first time based on the results of the COMPASS trial,11 which equated the choice between the 4 drugs. This is probably due to the results of the DAPT and PEGASUS-TIMI-54 studies, which found no evidence on the long-term efficacy of prasugrel. However, the guidelines do not comment on which drug would be preferable or on the duration of this strategy.
In patients with atrial fibrillation and an anticoagulation indication undergoing PCI, direct acting oral anticoagulants—at full doses with proven efficacy in stroke prevention—are now preferred to vitamin K antagonists (VKAs). However, the rivaroxaban dose used in the PIONEER-AF study was 15mg/d and there was also no significant reduction in the incidence of thromboembolic events with dabigatran 150mg/12h vs 110mg/12h but increased bleeding risk with the highest dose. Although somewhat controversial, triple antithrombotic therapy should also be maintained in the periprocedural period and during hospital admission. After hospital discharge, aspirin withdrawal (maintaining oral anticoagulation and clopidogrel) should be considered if the bleeding risk persists (new recommendation, IIa B). In patients with CCSs and high risk of stent thrombosis (previous stent thrombosis episode, suboptimal stent implantation, stent length> 60mm, diabetes, chronic kidney disease, bifurcation with 2 stents implanted, stent treatment of chronic occlusion) or with high risk of death if thrombosis occurs (stenting of the left main coronary artery, proximal descending artery, or last remaining patent vessel), the triple antithrombotic therapy may be extended (IIa C) beyond hospital discharge (1-6 months depending on the ischemic/bleeding risk balance). Aspirin should also be discontinued ≤ 1 week after PCI if the risk of stent thrombosis is low or the bleeding risk is very high. Similarly, oral anticoagulation should be stopped 12 to 48h before an elective PCI. Although the guidelines include the possible combination of prasugrel or ticagrelor with oral anticoagulants instead of triple antithrombotic therapy in patients with moderate or high risk of stent thrombosis, the authors correctly state that the evidence supporting this strategy is scarce.
Regarding other aspects of secondary prevention, the guidelines recommend that low-density lipoprotein (LDL-C) cholesterol levels be reduced below 1.4 mmol/L (55mg/dL) in patients with a very high risk of cardiovascular events, such as those with established ischemic heart disease.
REVASCULARIZATIONCompared with the previous guidelines,12 there is a major conceptual change regarding the role of revascularization in stable patients: revascularization is now the treatment of choice for these patients. The current document recommends a more liberal use of coronary revascularization, based primarily on the FAME 2 trial.13 This study found that coronary revascularization improved quality of life and reduced the use of antianginal medication vs medical therapy in patients with coronary stenosis and an FFR <0.80. The long-term results (5 years) of this study showed a benefit of angioplasty vs medical therapy, with lower rates of urgent revascularization and AMI. Similar results have been observed in meta-analyses,14 with revascularization with angioplasty or surgery reducing the rate of death and infarction vs medical therapy. In addition, another meta-analysis found that FFR-guided angioplasty reduced the risk of infarction or death by 26% vs medical therapy in patients with stable coronary heart disease.
The guidelines do not mention the role of FFR-guided surgical revascularization, even though one of the benefits of this technique is protection against proximal disease progression that affects distally assessed hemodynamic parameters. The authors note that not all of the evidence favors revascularization in this context because the ORBITA study, which included a sham procedure in the control group, found no significant improvement in exercise capacity with angioplasty vs medical therapy. However, the study had a small sample size and short follow-up time.
Finally, the guidelines recommend individualized risk/benefit analysis when myocardial revascularization is being considered and highlight the value of consensual decision-making. In contrast to the previous guidelines, the current document does not address the debate on the preferences for revascularization, angioplasty, or coronary surgery, use of the new antiplatelet agents, and use of surgical risk (EUROSCORE) or coronary disease complexity (SYNTAX SCORE) scales and refers to the relevant guidelines.
In summary, the guidelines advise a less restrictive use of myocardial revascularization in patients with stable coronary disease and do not limit the technique to a specific anatomy or ischemia extent (> 10%), but also allows the approach for patients with coronary stenosis with an FFR <0.80 in main coronary arteries.
Patients with new-onset heart failure or reduced left ventricular functionThe guidelines describe new presentations of CCSs. In patients with new-onset HF with left ventricular dysfunction, the recommendations are to assess LVEF via echocardiography, to determine natriuretic peptide levels, and to adhere to HF guidelines.14
Follow-up of patients with chronic coronary syndromesClinical variables and biomarkers should be periodically assessed in patients with CCSs. Two groups are distinguished: those who have had an ACS or revascularization less than 1 year before and those who have had an ACS more than 1 before. For the former, at least 2 visits are advised: ECG and echocardiography after revascularization. For the latter, the guidelines stress the periodic reassessment of symptoms, cardiovascular risk, and new comorbidities, annual follow-up with laboratory tests and ECG, and echocardiography every 3 to 5 years. CT coronary angiography may be useful in patients with surgical coronary revascularization. To assess silent ischemia, a stress imaging test can be performed every 3 to 5 years. The use of echocardiography to evaluate silent ischemia in patients with CCSs is debatable because it is not included in other guidelines15 and could saturate the healthcare system without providing major benefits for patients. Invasive coronary angiography is reserved for symptomatic patients who are refractory to treatment or have high risk.
Angina without obstructive disease in the epicardial coronary arteriesA new chapter is dedicated to the management of patients with angina without evidence of significant stenosis in the epicardial coronary arteries. However, there are no comments on the more frequent presence of this entity and its inadequate documentation in women. Two conditions are described: microvascular angina and vasospastic angina. Regarding the former, it is important to determine its cause, differentiating between impaired coronary microcirculatory conductance (documented by an FFR decrease or microcirculatory resistance index abnormalities) and an arteriolar endothelial dysregulation (documented by a positive acetylcholine test). According to the CorMiCa study,16 the treatment response differs according to pathophysiological mechanism: if the FFR is reduced but the acetylcholine provocation test is normal, treatment should comprise beta-blockers, angiotensin-converting enzyme inhibitors, and statins, whereas if the test is abnormal, treatment should entail nitrates and calcium antagonists. In addition, a microcirculatory disorder indicated by reduced FFR values is associated with poor prognosis that is comparable to that of patients with significant coronary stenosis. Therefore, quantification is recommended (IIa) of FFR or coronary microcirculatory resistance in patients with angina but without anatomically or functionally significant coronary lesions. This conceptual change will be difficult to include in clinical practice in Spain because the technique is not performed in our catheterization laboratories except in very specific centers. In addition, weight loss and lifestyle changes are highlighted as essential elements of treatment.
The need to definitively diagnose vasospastic angina via intracoronary provocation tests (acetylcholine or ergonovine, IIa indication) is emphasized, as well as the low risk of ventricular arrhythmic events. Intravenous infusion is not recommended due to the risk of vasospasm in the entire coronary tree. The recommended therapy entails calcium antagonists and long-acting nitrates.
Screening for coronary artery disease in asymptomatic patientsThe SCORE system should be used for the risk stratification of asymptomatic patients. Similarly, 2 interventions with recommendation level I are advised: evaluation of the first-degree family history of individuals with early ischemic heart disease (men younger than 55 years and women younger than 65 years) and, in the case of individuals younger than 50 years, clinical screening for familial hypercholesterolemia.
CT quantification of coronary calcification without contrast medium or ultrasound detection of carotid or femoral plaque can be considered risk modifiers (IIb B). CT coronary angiography or functional imaging may be indicated (IIb C) for the detection of asymptomatic CAD in patients with high risk of atherosclerotic cardiovascular disease and without known CAD.
Chronic coronary syndromes in specific circumstancesThe guidelines incorporate the differential management of 2 groups of patients with CCSs with special characteristics and make the following recommendations:
- •
In patients with cardiovascular comorbidities such as a) hypertension: optimal control of blood pressure (BP), namely, 120 to 130mmHg (130 to 140mmHg in those older than 65 years); b) valve surgery (including transcatheter aortic valve implantation [TAVI]): to evaluate previous CAD, invasive coronary angiography is the first-line test, particularly in men> 40 years and postmenopausal women with at 1 least cardiovascular risk factor, whereas CT coronary angiography is an alternative in patients with low CAD risk; c) heart transplant: annual invasive coronary angiography during the first 5 years and every 2 years thereafter.
- •
In patients with noncardiovascular comorbidities, such as a) cancer: the authors highlight the association of CAD with radiotherapy to the mediastinum, immunotherapies, and cardiotoxic chemotherapy); b) diabetes mellitus: sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists are recommended, as well as an LDL-C reduction ≥ 50% from baseline or to <1.4 mmol/L (< 55mg/dL); c) chronic kidney disease: there appears to be a linear relationship between CV risk and a greater reduction in glomerular filtration and the authors note the elevated risk of CAD and value of invasive treatment; d) elderly patients: the guidelines advise evaluation of comorbidities, frailty, and life expectancy and, in the case of an invasive approach, radial access, the use of drug-eluting stents, and short DAPT; e) women: the authors highlight their poor representation in clinical studies, the atypical nature of their symptoms, and the elevated risk in women older than 60 years and after revascularization, as well as the inability of hormone replacement therapy to reduce CAD risk in menopausal women (III C); and f) refractory angina: these patients should be treated in specific multidisciplinary teams and the guidelines stress the role of external counterpulsation and, as a novelty, coronary sinus reduction (IIb B).
This section includes the 19 key messages of the guidelines, already discussed in the corresponding sections of the document. The notable messages include: a) the appropriate use of noninvasive and invasive diagnostic tests for the diagnosis of ischemic heart disease; b) the value of secondary prevention that includes pharmacological and nonpharmacological aspects, stressing the usefulness of lifestyle changes; c) an individualized approach to drug treatment strategies, which is especially important for antithrombotic agents; and d) the relevance of a long-term follow-up of these patients, focusing on treatment adherence, healthcare education for patients, and the detection of potential changes in patients’ risks and comorbidities.
Gaps in the evidenceRegarding diagnosis, estimation of the pretest probability needs to be improved through biomarkers, imaging tests, and evaluation of other risk factors/comorbidities, extending this diagnostic approach to asymptomatic patients (as a screening strategy).
In terms of treatment, the optimal medical therapy for these patients remains to be defined. It is not yet clear whether beta-blockers have a prognostic value in patients with preserved LVEF who have already had an infarction. In this regard, several clinical trials are already underway; for example, a large clinical trial has started in Spain with more than 60 participating hospitals (REBOOT trial, NCT03596385).
It is also not clear whether antianginal treatment with first-line drugs is superior to that with second-line drugs (with or without combination). Furthermore, there are gaps in the evidence for the best treatment used for refractory angina.
Regarding antithrombotic therapy, it remains to be determined whether the combination of aspirin with a P2Y12 inhibitor is superior to the combination of aspirin with rivaroxaban, instead of treatment with long-term aspirin monotherapy.
The effect of complete coronary revascularization on prognosis remains to be consistently demonstrated according to type of revascularization (percutaneous or surgical) and the guideline followed (anatomical or functional).
What to do and what not to doThis interesting section summarizes the class I (recommended/indicated) and III (not recommended) indications of the guidelines. Notably, of a total of 103 theoretically clear recommendations on what to do (87) and not to do (16), only 38.8% have level of evidence A, whereas 22.3% and 38.8% have levels of evidence B and C, respectively. This aspect indicates that there are still numerous gaps in the available evidence and that more studies are required to provide relevant information for the treatment of this disease.
To conclude, the main novelties regarding the guideline recommendations on what can and cannot be done are as follows:
- •
CT coronary angiography should be the initial test in patients with suspected obstructive CAD.
- •
Direct acting oral anticoagulants are preferred to VKAs in patients with atrial fibrillation.
- •
Proton pump inhibitors are indicated for patients with high bleeding risk who are taking antiplatelet agents or anticoagulants.
- •
Ezetimibe or PCSK9 inhibitors should be combined with statins when LDL-C targets are not achieved.
- •
SGLT2 inhibitors or GLP-1 receptor agonists should be used in diabetic patients with cardiovascular disease.
- •
Carotid intimomedial thickness should not be measured to modify cardiovascular risk.
No conflicts of interest have been declared in relation to the present work.
SEC Working Group for the 2019 ESC guidelines on chronic coronary syndromes: Antonia Sambola (coordinator), Borja Ibáñez (coordinator), Rut Andrea, Gemma Berga, José Antonio Blázquez, Victoria Delgado, José Luis Ferreiro, Felipe Navarro, Sergio Raposeiras-Roubin, and Rafael Rodríguez Lecoq.
Expert reviewers for the 2019 ESC guidelines on chronic coronary syndromes: Albert Ariza Solé, Manuel Barreiro Pérez, Esteban López de Sá, Amparo Martínez Monzonis, Raúl Moreno, Carolina Ortiz, Armando Pérez de Prado, and Javier Torres Llergo.
SEC Guidelines Committee: Fernando Arribas, Gemma Berga Congost, Héctor Bueno, Arturo Evangelista, Ignacio Ferreira-González, Manuel Jiménez Navarro, Francisco Marín, Leopoldo Pérez de Isla, Antonia Sambola, Rafael Vázquez, Ana Viana-Tejedor, Borja Ibáñez, and Fernando Alfonso.