The guidelines for the diagnosis and treatment of acute and chronic heart failure (HF) for 2021 are an update on the previous guidelines from 5 years previously (2016).1,2 These guidelines have been eagerly anticipated due to the need to respond with practical recommendations to the high number of scientific publications that have since been released. However, the lack of agility of these guidelines is precisely their main weakness. Thus, the document has unfortunately not considered the simultaneous publication of new evidence on the management of HF with preserved left ventricular ejection fraction (LVEF) (HFpEF). Although the guidelines are a scientific tool for improving the diagnosis and treatment of our patients, continuous updating of their recommendations is clearly required to keep them current.
The purpose of the present article is to highlight the most relevant novelties of the new guidelines and to comment on those aspects that, from a Spanish perspective, could improve understanding and local application. To do so, we address various sections of the text and include table 1 and figure 1 as summaries.
Notable aspects of the recommendations and comments.
| Section | Relevant aspect | Comment |
|---|---|---|
| Definitions | Reduced LVEF, ≤ 40%Mildly reduced LVEF, 41%-49%Reduced LVEF, ≥ 50% | The concept of rEF is broadened and the mid-range form is now mrEF |
| HF diagnosis | pEF also requires structural or functional heart disease that indicates diastolic dysfunction or increased filling pressure (ultrasound or natriuretic peptides) | This requirement only applies to pEF (also previously applied to mid-range)Elevated natriuretic peptides should always be included in the diagnosis of pEFThere is no reference to lung ultrasound |
| Etiological and phenotypic characterization | The guidelines stress the study of etiologies and comorbidities in nonischemic phenotypes and in pEF. Special attention to cardiomyopathies (genetic) and amyloidosis | It would have been useful to broaden the recommendations regarding CMR. Confusing criteria in the indication for coronary CT angiography and invasive coronary angiography |
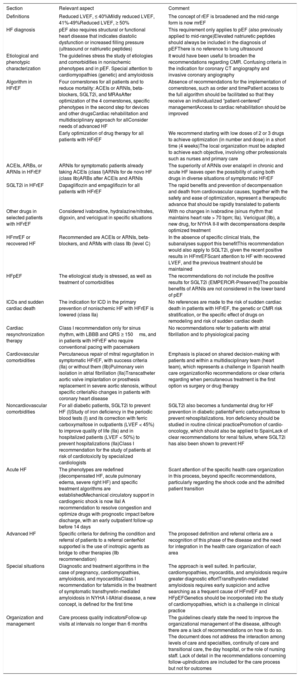
| Algorithm in HFrEF | Four cornerstones for all patients and to reduce mortality: ACEIs or ARNIs, beta-blockers, SGLT2i, and MRAsAfter optimization of the 4 cornerstones, specific phenotypes in the second step for devices and other drugsCardiac rehabilitation and multidisciplinary approach for allConsider needs of advanced HF | Absence of recommendations for the implementation of cornerstones, such as order and timePatient access to the full algorithm should be facilitated so that they receive an individualized “patient-centered” managementAccess to cardiac rehabilitation should be improved |
| Early optimization of drug therapy for all patients with HFrEF | We recommend starting with low doses of 2 or 3 drugs to achieve optimization (in number and dose) in a short time (4 weeks)The local organization must be adapted to achieve each objective, involving other professionals such as nurses and primary care | |
| ACEIs, ARBs, or ARNIs in HFrEF | ARNIs for symptomatic patients already taking ACEIs (class I)ARNIs for de novo HF (class IIb)ARBs after ACEIs and ARNIs | The superiority of ARNIs over enalapril in chronic and acute HF leaves open the possibility of using both drugs in diverse situations of symptomatic HFrEF |
| SGLT2i in HFrEF | Dapagliflozin and empagliflozin for all patients with HFrEF | The rapid benefits and prevention of decompensation and death from cardiovascular causes, together with the safety and ease of optimization, represent a therapeutic advance that should be rapidly translated to patients |
| Other drugs in selected patients with HFrEF | Considered ivabradine, hydralazine/nitrates, digoxin, and vericiguat in specific situations | With no changes in ivabradine (sinus rhythm that maintains heart rate > 70 bpm; IIa). Vericiguat (IIb), a new drug, for NYHA II-II with decompensations despite optimized treatment |
| HFmrEF or recovered HF | Recommended are ACEIs or ARNIs, beta-blockers, and ARMs with class IIb (level C) | In the absence of specific clinical trials, the subanalyses support this benefitThis recommendation would also apply to SGLT2i, given the recent positive results in HFmrEFScant attention to HF with recovered LVEF, and the previous treatment should be maintained |
| HFpEF | The etiological study is stressed, as well as treatment of comorbidities | The recommendations do not include the positive results for SGLT2i (EMPEROR-Preserved)The possible benefits of ARNIs are not considered in the lower band of pEF |
| ICDs and sudden cardiac death | The indication for ICD in the primary prevention of nonischemic HF with HFrEF is lowered (class IIa) | No references are made to the risk of sudden cardiac death in patients with HFrEF, the genetic or CMR risk stratification, or the specific effect of drugs on remodeling and risk of sudden cardiac death |
| Cardiac resynchronization therapy | Class I recommendation only for sinus rhythm, with LBBB and QRS ≥ 150ms, and in patients with HFrEF who require conventional pacing with pacemakers | No recommendations refer to patients with atrial fibrillation and to physiological pacing |
| Cardiovascular comorbidities | Percutaneous repair of mitral regurgitation in symptomatic HFrEF, with success criteria (IIa) or without them (IIb)Pulmonary vein isolation in atrial fibrillation (IIa)Transcatheter aortic valve implantation or prosthesis replacement in severe aortic stenosis, without specific criteriaNo changes in patients with coronary heart disease | Emphasis is placed on shared decision-making with patients and within a multidisciplinary team (heart team), which represents a challenge in Spanish health care organizationNo recommendations or clear criteria regarding when percutaneous treatment is the first option vs surgery or drug therapy |
| Noncardiovascular comorbidities | For all diabetic patients, SGLT2i to prevent HF (I)Study of iron deficiency in the periodic blood tests (I) and its correction with ferric carboxymaltose in outpatients (LVEF < 45%) to improve quality of life (IIa) and in hospitalized patients (LVEF < 50%) to prevent hospitalizations (IIa)Class I recommendation for the study of patients at risk of cardiotoxicity by specialized cardiologists | SGLT2i also becomes a fundamental drug for HF prevention in diabetic patientsFerric carboxymaltose to prevent rehospitalizations. Iron deficiency should be studied in routine clinical practicePromotion of cardio-oncology, which should also be applied to SpainLack of clear recommendations for renal failure, where SGLT2i has also been shown to prevent HF |
| Acute HF | The phenotypes are redefined (decompensated HF, acute pulmonary edema, severe right HF) and specific treatment algorithms are establishedMechanical circulatory support in cardiogenic shock is now IIaI A recommendation to resolve congestion and optimize drugs with prognostic impact before discharge, with an early outpatient follow-up before 14 days | Scant attention of the specific health care organization in this process, beyond specific recommendations, particularly regarding the shock code and the admitted patient transition |
| Advanced HF | Specific criteria for defining the condition and referral of patients to a referral centerNot supported is the use of inotropic agents as bridge to other therapies (IIb recommendation) | The proposed definition and referral criteria are a recognition of this phase of the disease and the need for integration in the health care organization of each area |
| Special situations | Diagnostic and treatment algorithms in the case of pregnancy, cardiomyopathies, amyloidosis, and myocarditisClass I recommendation for tafamidis in the treatment of symptomatic transthyretin-mediated amyloidosis in NYHA I-IIAtrial disease, a new concept, is defined for the first time | The approach is well suited. In particular, cardiomyopathies, myocarditis, and amyloidosis require greater diagnostic effortTransthyretin-mediated amyloidosis requires early suspicion and active searching as a frequent cause of HFmrEF and HFpEFGenetics should be incorporated into the study of cardiomyopathies, which is a challenge in clinical practice |
| Organization and management | Care process quality indicatorsFollow-up visits at intervals no longer than 6 months | The guidelines clearly state the need to improve the organizational management of the disease, although there are a lack of recommendations on how to do so. The document does not address the interaction among levels of care and specialties, continuity of care and transitional care, the day hospital, or the role of nursing staff. Lack of detail in the recommendations concerning follow-upIndicators are included for the care process but not for outcomes |
ARBs, angiotensin II receptor blockers; ACEIs, angiotensin-converting enzyme inhibitors; ARNIs, angiotensin receptor-neprilysin inhibitors; BBs, beta-blockers; CMRI, cardiac magnetic resonance imaging; CT, computed tomography; HF, heart failure; ICD, implantable cardioverter-defibrillator; LBBB, complete left bundle branch block; LVEF, left ventricular ejection fraction; MRAs, mineralocorticoid receptor antagonists; mrEF, mildly reduced LVEF; NPs, natriuretic peptides; pEF, preserved LVEF; rEF, reduced LVEF; SGLT2i, sodium-glucose cotransporter-2 inhibitor.
Schematic representation of the main recommendations and comments and their implementation by LVEF phenotype. Green, ESC class I; yellow, ESC class IIa; orange, ESC class IIb; gray, guideline comments. ACEIs, angiotensin-converting enzyme inhibitors; ARNIs, angiotensin receptor-neprilysin inhibitors; CMRI, cardiac magnetic resonance imaging; CRT, cardiac resynchronization therapy; Fe, iron; ICD, implantable cardioverter-defibrillator; HF, heart failure; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MRAs, mineralocorticoid receptor antagonists; mrEF, mildly reduced LVEF; NYHA, New York Heart Association functional class; pEF, preserved LVEF; PVs, pulmonary veins; rEF, reduced LVEF; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SR, sinus rhythm; TTR, transthyretin.
The new guidelines adopt the recent universal definition of HF, agreed upon by the scientific bodies of the European, North American, and Japanese cardiology societies.3 This definition continues to assign a key role to LVEF. The following aspects stand out: a) HF with reduced LVEF (HFrEF) is reestablished at LVEF ≤ 40%, vs the previous definition of < 40%, an apparently small change that may have a major impact on moving more patients toward the rEF phenotype and its treatments; b) HF with midrange LVEF is now named mildly reduced (HFmrEF) and is defined by a LVEF from 41% to 49%, another simple terminology change that moves these patients to “mild HFrEF” and closer to its treatments; c) HFpEF is maintained at LVEF ≥ 50% but, given its variability, must be interpreted in terms of the clinical context of each patient and still requires evidence of structural or functional changes, either by echocardiography or natriuretic peptides (NPs); NP elevation is included in the definition of HFpEF and removed from that of HFmrEF, although its unequivocal need is not established; in our opinion, elevated NP levels should be essential for the definition of HFpEF; d) the guidelines highlight acute HF and advanced HF as relevant entities that should be managed separately from chronic HF, given that they require distinct therapeutic approaches; e) there continues to be a discordance between the definitions based on LVEF and the cutoff points for therapeutic decisions that should be standardized; f) HF with “recovered” LVEF receives scant attention, despite being a fourth HF group in the consensus of the first universal definition of HF3; it concerns patients with HF and baseline LVEF ≤ 40% who show a ≥ 10-point increase in LVEF with a second measurement > 40%; g) the guidelines continue to recognize the usefulness of the New York Heart Association (NYHA) classification in treatment decisions, despite its limited objectivity, due to its use in the design of clinical trials; strikingly, the therapeutic recommendations are all aimed at patients in NYHA II-IV and neglect the reality of patients with HF in NYHA I; and h) a phenotype not addressed in these guidelines is that of patients with de novo HF, who deserve attention to strengthen the need to optimize the etiological study and the therapeutic approach as soon as possible after the initial diagnosis.
DIAGNOSIS OF HEART FAILUREThe algorithm is the same as before, based on signs and symptoms, NPs, and echocardiography.
The following are the 4 most relevant aspects: a) the document stresses NP measurement as a preliminary step before echocardiography to rule out the presence of HF, and the previous cutoff values remain; this places the European guidelines ahead of the North American guidelines, which do not provide reference values; b) a simplified approach is established for the diagnosis of HFpEF, based on the presence of diastolic dysfunction or elevated filling pressures (which includes elevated NPs); usefully, the various parameters are numbered (table 9 of the guidelines) and include elevated NP values specific for patients with atrial fibrillation (AF), which represents a novelty; other previously proposed diagnostic scores are not recommended; in doubtful cases, cardiopulmonary exercise testing or a diastolic stress test can be considered; a specific diagnostic algorithm would be useful for HFpEF to improve the characterization and accuracy of its diagnosis; c) recommendations on lung ultrasound must be incorporated to support the clinical diagnosis of pulmonary congestion or HF decompensation, particularly given its current rapid incorporation into clinical practice; and d) new parameters such as “myocardial deformation” and “ventricular mechanics” should be included in situations of doubt, but the guidelines do not expand on these situations.
ETIOLOGICAL CHARACTERIZATIONThis aspect becomes relevant in the 2021 guidelines, with the aim of individualizing specific therapeutic strategies. The most noteworthy aspects are the following: a) the genetic study of cardiomyopathies is included, with a well-formulated section and a list of mutations to be considered in our clinical practice (table 25 of the guidelines), a recommendation that poses a challenge regarding accessibility for the various professionals; b) the indications in cardiac magnetic resonance (CMR) are maintained from the previous guidelines, such as class I in cases of poor-quality echocardiography and in suspected infiltrative, inflammatory, or deposition disease; this limited recommendation does not seem in line with clinical practice and current evidence, given the usefulness of CMR in myocardial characterization and risk stratification; c) a class I recommendation is awarded to the screening of specific etiologies and comorbidities in HFpEF, a positive aspect, with special relevance for transthyretin amyloidosis; d) in the study of comorbidities, we must highlight the class I recommendation for the periodic studying of ferrokinetics, whose role in the setting of acute HF is strengthened and expanded; and e) the recommendations regarding the study of coronary heart disease are confusing, with invasive coronary angiography changed to a class IIb recommendation in intermediate-to-high–risk individuals and computed tomography coronary angiography changed to class IIa in low- or intermediate-risk individuals.
TREATMENT OF CHRONIC HEART FAILURETreatment algorithm in HFThis is one of the most relevant and groundbreaking aspects vs the previous guidelines. Stepwise and vertical drug therapy disappears, with the 4 cornerstones of treatment proven to reduce mortality and hospitalizations in patients with HFrEF placed in the same first horizontal step from initiation and with the same maximum recommendation (class I). These therapies are angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor-neprilysin inhibitors (ARNIs), beta-blockers, mineralocorticoid receptor antagonists, and, as the main novelty, sodium-glucose cotransporter 2 inhibitors (SGLT2i), a treatment that must be optimized early and before consideration of other drug or device interventions.
Next, in the second step, the algorithm considers the different phenotypes of HFrEF, based on whether the etiology is ischemic or not, the presence of valve disease, the QRS morphology and duration, the presence of atrial fibrillation, and heart rate.
In the third step, the algorithm incorporates the group of patients with advanced HF criteria and reflects the importance of all of the professionals managing this stage of the disease. Their identification is thereby promoted, as well as access both to palliative care structures and referral centers with heart transplant facilities and short- and long-term circulatory assist devices.
Finally, the document correctly clarifies that all patients with HF should undergo cardiac rehabilitation and be managed using a multidisciplinary approach to reduce hospitalizations and improve quality of life, an aspect that will be a challenge for all types of health care systems, and particularly in Spain, where it remains a pending issue.
Early optimization of drug therapy in all patients with HFrEFIt seems reasonable to implement as soon as possible any drugs that improve survival and to move away from stepwise decision-making, which may represent a barrier for patients to the benefits of these treatments. The present guidelines remove the historic chronology of clinical trials for each drug group, which sustain the “drug to drug” and “wait and see” strategies.
Undoubtedly, this new proposal is closer to the needs of the disease and of the patient but practical implementation will still be a challenge. Indeed, the main related criticism in this regard is the absence of a proposal, even just generic, for the initiation of these drugs in terms of order and dose, particularly in patients with de novo HF. Nonetheless, the difficulty of such an approach must be recognized, given the variations among patients and even in the same patient.
Contemporaneously with the guidelines, a document was published providing a strategic focus based on clinical phenotypes,4 including blood pressure, heart rhythm and rate, renal function, electrolytes, and other comorbidities. Although not part of the guidelines, this consensus document complements their recommendations and is worth reading.
The European Society of Cardiology (ESC) recommends the use of individualized drug titration schemes based on outlined phenotypic considerations.4 In addition, in patients with de novo HF, we recommend initiation with low doses of 2 or 3 drugs to achieve early drug optimization (in terms of number and dose), which should take less than 4 weeks. To optimize the drug therapy as quickly as possible and obtain the maximum tolerated dose, joint and coordinated work among the different health care professionals involved is more necessary than ever. The early implementation of all cornerstones requires improvement in the organizational environment of the disease that will surely improve patient care. Only thus will we be able to successfully implement these recommendations.
ACEIs, ARBs, and ARNIs in patients with HFrEFA controversial aspect is the recommendation regarding valsartan/sacubitril therapy (ARNIs). The previous guidelines indicated ARNIs only in patients who remained symptomatic despite treatment with ACEIs or angiotensin II receptor blockers (ARBs). The new recommendations assign the same level to ACEIs and ARNIs (class I) but indicate less evidence for ARNIs (B) than ACEIs (A). The new guidelines do not quite recognize the superiority of ARNIs over enalapril in the PARADIGM study but, at the same time, leave the choice for each patient (ACEIs or ARNIs) up to the professional. However, the text contemplates initiation with ARNIs only in patients who are already taking ACEIs and remain symptomatic; thus, ARNIs receive a class IIb recommendation for initiation with previous ACEIs in de novo, ambulatory, or hospitalized patients. Even so, their administration to de novo patients could be considered, as already proposed in a previous document, also from the ESC.5
Finally, ARBs are surprisingly recommended (I B) in patients who do not tolerate ACEIs or ARNIs, hinting (without saying) that the alternative should be ARNIs ahead of ARBs in patients who do not tolerate ACEIs.
SGLT2i in patients with HFrEFUndoubtedly, the main therapeutic novelty of the guidelines and one of the major revolutions in HF in recent years is the prescription of SGLT2i to patients with HFrEF. Hypoglycemic drugs are the cornerstone of the prevention and treatment of HF. In patients diagnosed with HFrEF, independently of their glycemic status, dapagliflozin or empagliflozin are recommended to reduce HF hospitalizations and cardiovascular death (class I A), based on DAPA-HF and EMPEROR-Reduced outcomes and a subsequent meta-analysis,6 assuming a class effect of both molecules.
Dapagliflozin and empagliflozin are in the first step of the therapeutic algorithm, with the same level of recommendation as neurohormonal antagonists. Still, they are recommended in the text after optimization of treatment with the other pharmacological cornerstones, given that their benefit was shown in patients already taking them at recommended doses.6 However, the early benefits and other advantages, such as a single dosage, absence of titration, scant effect on blood pressure, and the wide margin of use by renal function (estimated glomerular filtration rate > 25mL/min/1.73 m2), probably facilitate their rapid implementation in clinical practice, with no need to wait for the other pharmacological cornerstones.
Other drugs in selected patients with HFrEFIvabradine, hydralazine/nitrates, and digoxin receive the same level of recommendation. An addition is vericiguat, a guanylate cyclase receptor stimulator, in patients in NYHA II-III and with HF deterioration despite optimized therapy (class IIb). However, its recommendation could have been higher (IIa), given that a clinical trial showed a significantly reduced rehospitalization risk in these patients.
Treatment of patients with HFmrEF and recovered HFThe introduction in 2016 of the then-new group with midrange LVEF has enabled study and understanding that their clinical characteristics, risk factors, and cardiac remodeling patterns are more similar to those of HFrEF than HFpEF. With all of this information, this new category has been reconfigured and plays a greater role, with its own section, and its treatment is now more similar to that of HFrEF. Thus, ACEIs, ARBs, beta-blockers, MRAs, and ARNIs receive class IIb and level C recommendations, supported by the subanalyses of randomized clinical trials for HFrEF, the clinical similarity of these patients and those with HFrEF, and the fact that many patients were previously considered to have had HFrEF. The publication of the EMPEROR-Preserved study with empagliflozin, which included patients in this mrEF range,7 should also support the use of SGLT2i for HFmrEF. Thus, as in HFpEF, it seems that the guidelines here are out of date.
There are no device-related recommendations in this group, although some studies have shown an elevated risk of sudden cardiac death when fibrosis is identified on CMR.8 Therefore, there is a gap in the understanding of HFmrEF that should be bridged using specifically designed studies for this group.
LVEF is a continuous variable, subject to intraobserver and interobserver variability, with major changes during the course of the disease in a significant proportion of patients. In this regard, recovered HF has special importance, even though the guidelines do not recognize it, and such patients should follow the specific treatment for HFrEF.
Treatment in patients with HFpEFRegarding treatment, beyond the emphasis on comorbidities, there are no major changes from the previous guidelines and the authors conclude that no treatment has been shown to reduce morbidity and mortality in HFpEF.
However, coincident with the new guidelines, the outcomes have been published of the EMPEROR-Preserved study,7 in which empagliflozin significantly decreased the primary outcome (cardiovascular death or HF hospitalization) in patients with HF and LVEF > 40%, independently of the presence or absence of diabetes. As mentioned above, the outcomes of this study should have influenced the recommendations in these patients, particularly considering the limited treatment options for HFpEF. Despite the considerable heterogeneity of HFpEF syndrome, the guidelines have also declined to include recommendations on neurohormonal antagonists, including ARNIs.
Implantable cardioverter-defibrillators and sudden cardiac deathThe new guidelines contain some important novelties with an unclear impact on clinical practice. For the primary prevention of sudden cardiac death in nonischemic heart disease, the recommendation for implantable cardioverter-defibrillators (ICDs) has been relegated from class I in 2016 to IIa in 2021. The DANISH study found no significant improvement in overall mortality9 and the experts have thus decided to reduce the recommendation. Accordingly, selection is required of those patients in primary prevention who can benefit from ICDs and who do not have coronary heart disease. However, the guidelines make no recommendations on risk markers, such as type of cardiomyopathy (sarcoidosis), genetic mutations (laminin, filamin C, and RMB20), or late gadolinium enhancement on CMR. The latter has been proven to be superior to a 35% LVEF cutoff and could reclassify the risk of arrhythmias in up to a third of patients.8 Age and comorbidities could also play a role. Indeed, the DANISH study showed a significant benefit in the subgroup of patients younger than 59 years.
In addition, the guidelines no longer mention the usefulness of drugs in the prevention of sudden cardiac death, even though the treatment algorithm states that drugs must be optimized before device selection.
Cardiac resynchronizationThis section also contains novelties, such as the relegation in the level of recommendation from class I to IIa in patients in sinus rhythm, with left bundle branch block, and a QRS from 130 to 149ms, a change based on lower evidence of a benefit in this subgroup in both clinical trials and meta-analyses. This means that the recommendation is class I A only in patients in sinus rhythm with left bundle branch block and a QRS ≥ 150ms.
The other I A recommendation is in patients with ventricular dysfunction (LVEF < 35%) who require conventional right ventricular pacing due to bradyarrhythmia or to atrioventricular node ablation (AVN) for AF control. This recommendation is relevant in situations not purely cardiological, because various specialists already use conventional pacing with pacemakers. Indeed, the pacing guidelines recommend (class I) echocardiography before conventional pacemaker implantation.10 Similarly, the recommendation also increases from IIb to IIa regarding a switch to biventricular pacing in patients who already have conventional pacing and develop HF with LVEF ≤ 35%.
In addition, and this is a tendency associated with patient empowerment, it would be the decision of patients to undergo implantation with an ICD with cardiac resynchronization therapy (CRT). The document advises individualization and shared decision-making (IIa) regarding the implantation of CRT-D or CRT-P devices, with consideration of other factors influencing the prognostic benefit.
There is no specific recommendation in the presence of AF but resynchronization may be considered if the QRS is > 150ms and a high percentage (>95%) of biventricular pacing can be ensured. In addition, no comment is made on physiological pacing of the left bundle branch as an alternative to a coronary sinus lead, which nonetheless does appear in the European Society of Cardiology guidelines on pacing, published around the same time.10
CARDIOVASCULAR AND NONCARDIOVASCULAR COMORBIDITIESThe new guidelines pay renewed attention to comorbidities and have correctly decided to separate cardiovascular and noncardiovascular comorbidities into different sections.
The following are the 4 most notable novelties related to cardiovascular comorbidities: a) after conflicting clinical trials, the document recommends percutaneous edge-to-edge repair of secondary mitral regurgitation in patients with symptomatic HFrEF to prevent hospitalization with a class IIa indication in those who meet success criteria and IIb in those who do not; notably, the criteria of the COAPT study are included for candidate selection11 but, strikingly, the surgical option continues to be favored, despite the lack of data supporting mitral valve surgery alone in these patients; b) participation in multidisciplinary teams (heart teams) and shared decision-making with patients with severe aortic stenosis and rEF: the algorithm is updated in the presence of rEF and low-gradient stenosis and the choice between valve replacement and transcatheter aortic valve implantation (TAVI) is left to shared decision-making; in this regard, a desirable inclusion would be clearer recommendation criteria that include surgical risk, as in the new guidelines on valvular heart diseases12; c) pulmonary vein isolation in patients with AF and HFrEF receives a limited IIa recommendation, in contrast to the recent guidelines on atrial fibrillation, which indicate a class I recommendation; undoubtedly, this rhythm control option should be considered at early stages but should also be individualized, given the difficulty of generalizing the approach, due to logistic reasons and the patient selection bias in the clinical trials; and d) with no novelties concerning patients with coronary heart disease, the treatment of choice continues to be coronary revascularization in symptomatic patients despite optimal medical therapy, particularly in the presence of diabetes or multivessel disease (IIa); in all patients, individualization is required, considering coronary anatomy, comorbidities, and surgical risk.
The noncardiovascular comorbidities include 4 relevant novelties: a) SGLT2i for all patients with diabetes: the abundant evidence from studies with diabetic populations has led to a class I A recommendation in all diabetic patients with high cardiovascular risk or established cardiovascular disease to prevent progression to HF; b) ferric carboxymaltose in outpatients and admitted patients with HF: determination of iron deficiency is consolidated as a fundamental part of the assessment of patients with HF (I C); while the previous guidelines recommended ferric carboxymaltose in outpatients with LVEF < 45% and iron deficiency to improve symptoms and functional capacity (IIa A), the new guidelines also recommend this treatment for admitted patients with LVEF ≤ 50% to ameliorate symptoms and reduce readmissions (IIa B), based on the outcomes of the AFFIRM trial13; c) specialized care in patients with cancer and risk of cardiotoxicity: a strong recommendation (class I) is made that at-risk patients due to their history, risk factors, or cardiotoxic agents be previously assessed by cardiologists specialized in cardio-oncology, an important recommendation that, in Spain, should be an impetus in this area, the training of professionals, and the organization of care for these patients. The document also supports with a class IIa recommendation ACEIs and beta-blockers in patients who develop ventricular dysfunction (LVEF reduction > 10 points and < 50%) during treatment; and d) there are no recommendations on renal failure despite its relevance in HF; in addition, recent favorable results obtained with SGLT2i should have allowed an indication for the prevention of HF in patients with renal failure and microalbuminuria.
ACUTE HEART FAILUREThe diagnostic algorithm for acute HF (AHF) is largely unchanged and the main novelty lies in treatment based on pathophysiological phenotypes together with the establishment of 3 phases: immediate, intermediate, and predischarge and early follow-up (figure 11 of the guidelines). Notable novelties in the management of AHF include: a) treatment algorithms for the 4 new phenotypes: decompensated HF, acute pulmonary edema, right HF, and cardiogenic shock (figures 7-10 of the guidelines); b) the intensive use of intravenous loop diuretics guided by urine sodium and diuresis, before the use of combinations of other diuretics (figure 13 of the guidelines); we must highlight the reference to the use of carbohydrate antigen 125 to guide diuretic treatment, based on the contributions of Spanish groups in recent years; c) the routine use of opioids is discouraged, the level of recommendation is lowered for intravenous vasodilators after neutral clinical trials (from IIa to IIb), and norepinephrine is recommended instead of adrenaline in patients with cardiogenic shock requiring vasoconstrictor support; d) the level of recommendation is increased from IIb to IIa C for mechanical circulatory support in cardiogenic shock; and e) particularly relevant are the class I recommendations regarding the predischarge need for, first, the evaluation and treatment of signs of congestion, second, the initiation and optimization of oral disease-modifying treatment (cornerstones in HFrEF), and, finally, the planning of an early revision 12 weeks after discharge.
The document recognizes the importance of the time to admission not only for resolving the acute event, but also for improving disease prognosis in the short- and long-term. However, there are no recommendations on how to organize the care transition among professionals and care levels. There is also no reference to the organization of regional care for cardiogenic shock through multidisciplinary teams and referral centers. Recent Spanish results have shown the feasibility of a shock code and improved prognosis when treatment takes place in cardiac intensive care units led by cardiologists.14
ADVANCED HEART FAILUREIn the field of advanced HF, we must highlight the recognition of this phase of the disease with its inclusion in the main treatment algorithm and its definition with clear criteria. Taken together, the aim is to promote its organization and early referral to referral centers. Heart transplant continues to be the first option in advanced HF (I A), and the outcomes with new long-term mechanical circulatory assist devices have led to a IIa recommendation in candidates for either bridge to transplantation or destination therapy. The level of recommendation is reduced to IIb for inotrope infusion as bridge to transplantation, although no reference is made to their use as palliative care, a routine indication in Spain. In addition, the document stresses the value of the INTERMACS classification in risk stratification and decision-making.
SPECIAL CONDITIONSThe guidelines address various situations in this new section and highlight the circumstances or etiologies influencing diagnosis, risk stratification, and prognosis. The following aspects are notable: a) a useful algorithm is included for the management of pregnant women with HF but there is no specific section on sex differences in the disease; b) algorithms are presented for the study of myocarditis and cardiomyopathies, which include very useful criteria for diagnosis, phenotyping, and genotyping to improve characterization, a critical step toward specific treatments; c) an important therapeutic novelty, the section dedicated to amyloidosis, where treatment is recommended (class I B) for the first time with tafamidis in patients with transthyretin-mediated amyloidosis (hereditary or wild-type) in NYHA III; consequently, recommendations regarding the suspicion and diagnosis of this entity are even more important, with their underdiagnosis possibly limiting the understanding and prognosis of these patients; in this regard, multidisciplinary suspicion and diagnostic protocols are required in Spain, particularly in patients with HFmrEF or HFpEF and “warning signs”; and d) in a novel and interesting approach, atrial disease or myopathy is defined for the first time as a structural, electrophysiological, or functional change with clinical consequences, which primarily includes HFpEF and AF, an area of investigation in the coming years that should be driven by the guidelines.
ORGANIZATION AND MANAGEMENTThe guidelines represent a direct and indirect impetus to the need for management and improved organizational care of the disease. Thus, the document clearly states that patients with HF must be managed by multidisciplinary teams that integrate patient self-care and home-based and clinic-based care programs. In addition, the recommendation for rehabilitation is the highest possible (I A) in patients who can exercise but should also be considered in those with more severe disease, frailty, or comorbidities (IIa). Moreover, the guidelines strengthen the need for organizational care built around processes such as cardiogenic shock, advanced HF, or cardio-oncology.
However, the guidelines show deficiencies in their ability to provide recommendations on how to implement this organizational need in clinical practice. These shortcomings include the following: a) the section on chronic HF monitoring is nonspecific and indicates intervals no longer than 6 months, preferably by specialists, a concept incompatible with the need for a multidisciplinary and patient-focused approach that seeks complete therapeutic optimization; b) the guidelines award the maximum recommendation to an early postdischarge follow-up but do not address the concept of transition as a specific process that also requires a multidisciplinary approach and that would support the usefulness of the early visit; c) given that the management of HF involves other specialists, such as primary care physicians and internists, it would be useful to include a proposal for interactions among specialists and levels of care, as well as continuity of care during the disease; this proposal should be initiated at diagnosis, be included in the therapeutic optimization, and be adapted to each health care area; d) the recommendation for telemedicine is IIb, based on discordant results, but does not take into account the current situation and the role that it has acquired in the post-COVID era; e) there is no reference to day hospitals as a fundamental part of the care of patients with HF and, in particular, for the outpatient resolution of decompensations; f) no specific recommendation is made regarding the role of nurses in the different phases of the disease, which is sufficiently important to warrant a specific section addressing their participation in the diagnosis, care, and treatment of patients with HF, including drug titration; we must also insist on the need for the training of nurses specialized in HF, which remains unfinished business in the Spanish health care system; and, finally, g) notably, and for the first time, a final table (table 37 of the guidelines) is included with a list of quality indicators for the care process, which should also include outcome indicators.
CONCLUSIONSThe new guidelines represent an organizational challenge that should allow not only the implementation of the recommendations in clinical practice, but also promote a general improvement in the management of both the overall disease and each particular patient. This would be a challenge for all and for the Spanish health care system, whose motor is undoubtedly our scientific society itself.
FUNDINGNone.
CONFLICTS OF INTERESTThe conflict of interest declaration documents of all authors can be seen in the supplementary data.
SEC Working Group for the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Domingo Pascual Figal (coordinator), José Ramón González-Juanatey (coordinator), Antoni Bayes-Genis, Marta Cobo, Juan Delgado, Beatriz Diaz-Molina, José González Costello, Silvia López-Fernández, Rafael Mesa Rico, Julio Núñez Villota, Alfonso Valle, and José Luis Zamorano.
SEC Guidelines Committee: Pablo Avanzas, Gemma Berga Congost, Araceli Boraita, Héctor Bueno, David Calvo, Raquel Campuzano, Victoria Delgado, Laura Dos, Ignacio Ferreira-Gonzalez, Juan José Gomez Doblas, Domingo Pascual Figal, Antonia Sambola, Ana Viana Tejedor, José Luis Ferreiro (copresident), and Fernando Alfonso (copresident).
The names of all of the authors of this article are listed in alphabetical order in the appendix 1.
Corresponding author.
E-mail addresses: dpascual@um.es (D. Pascual Figal). jose.ramon.gonzalez.juanatey@sergas.es (J.R. González-Juanatey).