The new guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation include 131 recommendations, 3 fewer than the previous document, published in 2016, and a lower number of absolute recommendations (70% class I recommendations and 8% class III). Surprisingly, despite the accumulation of scientific evidence, there was a slight increase in recommendations with level of evidence C (43%).1 The document lists 16 novelties that can be summarized in 7 major changes, although there are no particularly radical amendments. The authors attempt to correct the error in the previous guidelines regarding the serial measurement of high-sensitivity troponin (hs-cTn T/I at 1 or 3hours)2 and now recommend the first-line use of the 0 hour/1 hour algorithm. This controversy probably makes little sense in Spain, given that the timing of the serial measurements varies and depends on laboratory pressure. Coronary computed tomography (CT) angiography has acquired a major diagnostic role in low-risk patients. Nonetheless, the most impactful changes affect antithrombotic therapy and revascularization strategy, with novelties that may cause controversy.
DEFINITIONS AND EPIDEMIOLOGYThe diagnosis of myocardial infarction (MI) is unchanged and revolves around the fourth universal definition.1 The routine use of hs-cTn T/I concentrations has resulted in a 4% absolute increase in the detection of MI and a decrease in the incidence of unstable angina. In Spain, about 40% of MIs are without ST-segment elevation.3
Diagnosis: symptoms, ECG, troponins, and imaging techniquesThe guidelines relegate medical history and physical examination to the supplementary material,1 a worrying sign at a time when the prioritization of the value of complementary tests has undermined that of clinical features. At least in this supplementary material, the authors do warn about the high frequency of atypical symptoms, particularly in the elderly and women, as well as in patients with diabetes, chronic kidney disease, or dementia. In addition, the guidelines stress the low diagnostic usefulness of chest pain characteristics, which is why it is essential to include cardiovascular history and cardiovascular risk factors in the patient assessment.
The star complementary test continues to be electrocardiography (ECG), a simple, cheap, and accessible test. Not only does it need to be performed in the first 10minutes after patient arrival at the emergency department or contact with the prehospital care service, but also it must be immediately interpreted by a qualified physician that, in the case of prehospital care, can perform it remotely. It is important to remember that the electrocardiographic tracing can be normal in up to one-third of patients with non–ST-segment elevation acute coronary syndrome (NSTEACS). In addition, the document reiterates that acute occlusion of the circumflex artery does not cause ST-segment elevation in standard ECG leads2 and that the V7 to V9 leads must thus be used, as well as V3R and V4R to detect right ventricular MI.
Patients with left bundle branch block (LBBB) and a high clinical suspicion of myocardial ischemia should still be treated as for ST-segment elevation MI. However, the authors note that more than 50% of patients with chest pain and LBBB, as well as those with right bundle branch block, will ultimately receive a diagnosis other than MI. Moreover, this recommendation also applies to patients with previous LBBB.
Regarding blood tests, hs-cTn T/I measurement dominates due to its high sensitivity and specificity. It must be remembered that hs-cTn T/I values depend on variables such as age, sex, renal failure, and, of course, time since symptom onset. In addition, myocardial injury is not solely caused by ischemia, with other situations (eg, pulmonary embolism, chest trauma, myocarditis, heart failure) able to markedly elevate hs-cTn T/I concentrations. Measurement of hs-cTn T/I concentrations according to the 0 hour/1 hour algorithm as first-line approach and the 0 hour/2 hour algorithm as second-line, in addition to clinical findings and ECG, enables the identification of candidates for early discharge and outpatient management. In doubtful cases requiring observation, a third measurement at 3hours and an echocardiogram can be helpful.
In patients with no ischemic changes on ECG and normal hs-cTn T/I concentrations, an additional imaging test is recommended, either during admission or in outpatient care. Stress echocardiography is recommended over exercise ECG due to its higher negative predictive value (NPV), whereas cardiac magnetic resonance (CMR) can assist in the differential diagnosis of MI, myocarditis, and tako-tsubo syndrome due to its tissue characterization capabilities. Other techniques, such as single-photon emission computed tomography (SPECT), can be useful in the risk stratification of these patients. CT allows visualization of coronary arteries with a high NPV to rule out coronary heart disease, although it is less useful for ruling out NSTEACS in patients with previous coronary heart disease and has not yet been validated in patients who have undergone surgical or percutaneous coronary revascularization. CMR can be more useful than CT for excluding other causes of chest pain, such as pulmonary thromboembolism and acute aortic syndrome.
RISK STRATIFICATIONThe risk stratification of patients with NSTEACS is based on electrocardiographic indicators, the levels of certain biomarkers, and the use of clinical ischemic and bleeding risk scales.
The current guidelines, in contrast to previous editions, very elegantly illustrate the distinct electrocardiographic patterns of NSTEACS that are associated with worse prognosis or characteristic angiographic findings. The following 3 biomarkers are recommended for risk stratification: a) the hs-cTn T/I peak, which adds prognostic value in terms of short- and long-term mortality: the higher the peak, the higher the mortality risk; b) the glomerular filtration rate, essential for calculating the GRACE score; and c) natriuretic peptides, which provide additional prognostic information over the hs-cTn T/I concentration. The GRACE scale (IIa B) is still the recommended risk scale for the prediction of clinical events after a NSTEACS. In numerous observational studies, this scale has been proven to be superior to the subjective assessment of the treating clinician in the prediction of risk of death or MI after an ACS. The main novelty in the evaluation of bleeding risk is the recommendation to use the Academic Research Consortium for High Bleeding Risk (ARC-HBR) scale4 as an alternative to the CRUSADE and ACUITY scales (IIb B). However, the ARC-HBR has not yet been validated in clinical trials.
The authors are hesitant about supporting the use of scales to optimize the duration and intensity of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI) (IIb B). The DAPT (at 1 year) and PRECISE DAPT (at hospital discharge) scales add slight predictive value because they are based on studies not including prasugrel or ticagrelor and not including patients requiring long-term anticoagulation. Moreover, clinical trials have not been performed to support their use.
PHARMACOLOGICAL MANAGEMENTChoice of antithrombotic treatmentThis is probably one of the most controversial sections, given that the recommendations are based on a low level of evidence and show discrepancies with the atrial fibrillation (AF) guidelines.5 However, the authors insist that the treatment chosen must reflect patients’ ischemic and bleeding risks, which are based on patient characteristics, clinical presentation, comorbidities, comedication (eg, need for oral anticoagulants), and procedural aspects.
Clopidogrel is recommended only when prasugrel and ticagrelor are contraindicated or when the bleeding risk is unacceptably high (I C). A novel addition is that prasugrel is now the therapy of choice. This decision was based on the outcomes of the ISAR-REACT-5 trial: in patients with and without ST-segment elevation managed with an invasive strategy, prasugrel without pretreatment was superior to ticagrelor in significantly reducing ischemic risk without increasing bleeding complications.6 Nonetheless, this study is not free from controversy due to certain limitations: the absence of double-blinding and the largely telephone-based follow-up and because its benefits are mainly due to a decreased incidence of periprocedural MI.6
PretreatmentA highly relevant novelty with direct impact on our routine daily practice, currently an object of debate and controversy in the scientific community, is that, in patients who are to undergo an early invasive strategy, a second antiplatelet agent should not be added if the coronary anatomy is unknown, regardless of the drug used (III A). The change to the pretreatment indication is based on the lack of clinical trials supporting the benefits of pretreatment in NSTEACS, whether with clopidogrel, ticagrelor, or prasugrel, and due to the potent and rapid inhibition that is achieved with the administration of these last 2 drugs in the catheterization laboratory. Some studies have shown no benefit on ischemic risk in pretreated patients but higher bleeding risk. In addition, pretreatment administration can delay surgery in patients scheduled for such procedures or have a negative impact on patients with a final diagnosis other than NSTEACS. For patients who cannot be offered an early invasive treatment, pretreatment can be considered with a P2Y12 receptor inhibitor, depending on the bleeding risk (IIb C).
A novelty in peri-interventional anticoagulation is the recognition of unfractionated heparin as the drug of choice (I A) due to its safety, effectiveness, and low cost. Bivalirudin and enoxaparin now have a lower level of recommendation, according to the current evidence. Finally, the use of fondaparinux (I B) is recommended only in cases of medical therapy or when timely patient transfer to a center with angioplasty capability is impossible and must be complemented with an additional bolus of unfractionated heparin at the time of PCI. There are no changes from previous guidelines regarding glycoprotein (GP) IIb/IIIa inhibitors and cangrelor.
Maintenance antithrombotic treatmentThe guidelines recommend a 12-month DAPT regimen, irrespective of stent type. However, in specific scenarios, treatment duration can depend on bleeding and ischemic risk. Patients with high bleeding risk are defined for the first time: bleeding in the last month or those with elective, nondeferrable, surgery. DAPT (aspirin plus clopidogrel) is recommended for 1 month, followed by clopidogrel monotherapy. Aspirin and P2Y12 receptor inhibitor (IIa B) dosage schedules of 3 to 6 months (IIa A) are possibilities, as well as prolonged regimens> 12 months (with their level of recommendation upgraded from IIb A to IIa A), depending on patients’ bleeding and ischemic risk. The guidelines include a clear scheme for assessing high ischemic risk, which additionally considers coronary anatomy and type of PCI. Based on the results of the TWILIGHT trial,7 which included patients with low-to-intermediate ischemic risk and low bleeding risk, these patients could be treated with DAPT for 3 months, followed by aspirin up to 12 months.
For the first time, de-escalation of P2Y12 receptor inhibitors such as prasugrel or ticagrelor to clopidogrel (IIb A), as an alternative to the 12-month regimen, is possible in patients deemed unsuitable for this regimen, although this group of patients remains to be defined. Another possible option is the combination of rivaroxaban 2.5mg/12h with aspirin and clopidogrel (IIb B) in patients with high thrombotic risk and without major risk of severe or life-threatening bleeding, as well in patients with moderately elevated thrombotic risk. However, there is no scientific evidence indicating the superiority of this strategy over the combination of aspirin with ticagrelor or prasugrel (COMPASS trial).8
Antithrombotic therapy in patients on oral anticoagulantsUnfortunately, the table summarizing the treatment of patients with AF (CHAD2DS2-VASc scores ≥ 1 in men and ≥ 2 in women) includes numerous possible alternatives and recommendations, as well as somewhat of a discrepancy with the AF guidelines, which undermines the initial intention to clarify and simplify. These discrepancies reflect the shortage of meta-analyses and clinical trials of these paradigms and show that the recommendations are based on the consensus of experts with different opinions on this matter. Moreover, the term “new anticoagulants” is obsolete and should be replaced with “direct oral anticoagulants” (DOACs).
The main novelty in patients undergoing PCI or receiving medical therapy is the significant change in the duration of the triple therapy (oral anticoagulant plus aspirin plus clopidogrel) from the previous guidelines.1 In addition, the preference for DOACs is established at the dose recommended for stroke prevention (although lower doses were used in the PIONEER-FA trial9) over vitamin K antagonists (VKAs). The strategy of choice is a 1-week regimen followed by dual therapy, preferably clopidogrel and a NOAC, for 1 year (I A). However, the ESC 2020 guidelines for the management of AF,5 considers, quite rightly, this recommendation to be class I B due to the lack of meta-analyses and clinical trials proving its efficacy and safety. Subsequently, a single anticoagulant is recommended after 1 year (I B). The dual therapy can be shortened to 6 months in patients with high bleeding risk, whereas the triple therapy can be prolonged up to 1 month in patients with high ischemic risk (IIa C). Another novelty, and a difference from the AF guidelines,5 is the possibility of dual therapy with a NOAC plus prasugrel or ticagrelor as an alternative to triple therapy when the stent thrombosis risk is moderate or high, regardless of the stent used (IIb C).
Another controversial aspect is that no exceptions have been established to the withdrawal of antiplatelet agents at 1 year in patients with high ischemic risk due to clinical or anatomical characteristics or a complex intervention. In patients requiring anticoagulation with VKAs, the dosage schedule is the same as that for those treated with NOACs; in patients receiving medical therapy, the recommendation remains for dual therapy with an anticoagulant plus an antiplatelet agent, preferably clopidogrel, for at least 6 months (IIa C).
The basic management of bleeding in patients receiving antithrombotic therapy is unaltered but, in patients with severe bleeding being treated with dabigatran or rivaroxaban, the use of their specific antidotes is recommended for the first time: idarucizumab or andexanet-alpha, respectively (IIa B). In patients being treated with VKAs, the anticoagulant should be rapidly reversed using prothrombin complex plus vitamin K (IIa C).
Pharmacological management of ischemiaThe classic indications are maintained for the pharmacological management of ischemia. In patients with unknown ventricular function, the very early administration of beta-blockers is not advised, as in patients who have coronary spasm or have taken cocaine, which is maintained from the previous guidelines.
RISK STRATIFICATION FOR INVASIVE MANAGEMENTMajor changes have been made to the decision-making regarding revascularization timing, which is based on patients’ risk stratification. The guidelines recommend early invasive treatment in the first 24hours of admission for NSTEACS based on dynamic ST-segment changes, hs-cTn T/I concentrations, and a GRACE score> 140 points in order to minimize adverse events and improve survival (see figure 1 of the guidelines). Highly unstable patients require immediate invasive treatment (< 2hours). Because this approach is similar to that of ST-segment elevation myocardial infarction, centers without PCI should transfer patients to their referral center. Although well founded, this recommendation is difficult to systematically apply in Spain, given the small number of catheterization facilities available 24hours a day. The most efficient option for implementing this recommendation would be to include these patients in STEACS care networks.
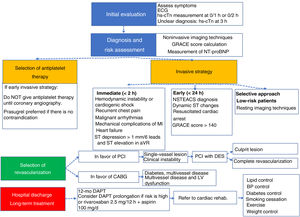
Novelties in the comprehensive management of non–ST-segment elevation acute coronary syndrome. BNP, B-type natriuretic peptide; BP, blood pressure; CABG, coronary artery bypass surgery; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEACS, non–ST-segment elevation acute coronary syndrome; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCI, percutaneous coronary intervention.
In these guidelines, in contrast to the previous document, the option has been removed for an “invasive strategy” such as coronary angiography/revascularization before 72hours. In low-risk patients, a selective invasive approach is required and a noninvasive stress test (preferably imaging) is recommended to detect inducible ischemia before an invasive strategy is chosen.2,10 The guidelines stress the need for an individualized assessment of the patient before ruling out coronary angiography because advanced age or logistical problems are insufficient reasons. Given that the high-risk group is highly likely to have recurrent ischemic events and cardiovascular mortality, coronary angiography must be restricted only in patients whose revascularization risk clearly outweighs the benefit. If the conservative option is chosen, the prescribed treatment and secondary prevention measures must be strictly followed. In patients with unstable angina, the guidelines recognize the limited benefit of PCI in the first 72hours.
The main technical aspects of PCI in patients with NSTEACS do not differ from those of the invasive evaluation and revascularization strategies for other manifestations of ischemic heart disease. Radial access is recommended (I A), and drug-eluting stents are already considered the standard treatment in PCI (I A). Given that NSTEACS usually involves multivessel disease and that there are no solid studies in this regard, in patients who are not in shock, the decision to treat the culprit vessel alone or to perform complete revascularization (PCI or CABG, according to SYNTAX score, etc), and whether this should be done in 1 procedure or in stages, must be based on the functional relevance of all stenoses, patient age, comorbidities, general clinical status, and left ventricular function (I B).
In patients requiring surgical revascularization, individualization is required of the timing of the procedure. The risk of an ischemic event related to suboptimal antiplatelet therapy is very low vs the bleeding risk in patients treated with antiplatelet drugs.
Unlike in the previous guidelines,2 and as a result of the COACT trial,11 coronary angiography can be delayed in patients resuscitated after out-of-hospital cardiac arrest. However, the evidence is scarce and future and ongoing studies will provide data for establishing the correct time of the intervention.
MYOCARDIAL INFARCTION WITH NONOBSTRUCTIVE CORONARY ARTERIES AND ALTERNATIVE DIAGNOSESThere is a new section on MI with nonobstructive coronary arteries (MINOCA), with the latest ESC definition12 replaced by that of the American Heart Association,13 which now excludes myocarditis and Takotsubo syndrome. An algorithm should be followed for its differential diagnosis (I C): CMR takes center stage by identifying the underlying cause in 85% of cases (I B); acetylcholine or ergonovine testing is recommended when vasospasm is suspected, and intravascular ultrasound or optical coherence tomography can be useful for the study of thrombus, plaque rupture, or spontaneous coronary artery dissection. In this last entity, a novel aspect is addressed, specifically, the algorithm analyzing the various therapeutic options: medical therapy and revascularization strategies, none supported by clinical trials (IIb C).
SPECIAL POPULATIONSThe presentation of NSTEACS as heart failure or cardiogenic shock is a diagnostic challenge. Its diagnosis relies on emergency coronary angiography and echocardiography. If a culprit lesion is found, the management—percutaneous (I B) or surgical if the coronary anatomy is not favorable (I B)—must be urgent. Overall, 80% of patients have multivessel disease, but PCI is not advised for nonculprit lesions based on CULPRIT-SHOCK14 trial outcomes (III B). The intra-aortic balloon pump is still not recommended unless there is a mechanical complication (III B). It remains to be determined whether other short-term circulatory support devices are associated with superior survival vs the balloon pump; Impella has even shown higher mortality. Nonetheless, they continue to receive a class IIb C indication pending the results of ongoing randomized trials.
Less space is devoted to populations with major impact on routine clinical practice, such as women, elderly patients, and patients with chronic kidney disease. The document notes that assessment of frailty and the risk-benefit balance must underlie therapeutic decisions. However, the management of cancer patients is not discussed, despite warranting special attention.
WOMEN AND NSTEACSSurprisingly, women are still included in “special populations”. Registry data3,15 demonstrate inequalities regarding access to evidence-based treatments and women continue to be underrepresented in clinical trials. The same recommendations as for the general population should be followed regarding coronary angiography, and drug dosage should be adjusted according to weight and glomerular filtration rate.
LONG-TERM MANAGEMENT OF NSTEACSMultidisciplinary management has acquired a major role in the treatment of this entity to reduce morbidity and mortality and improve quality of life. Psychosocial factors, mood disorders, stress, and anxiety have gained relevance due to their association with worse adherence to treatment and healthy lifestyles. Cognitive-behavioral interventions (I A) are necessary to help individuals to achieve healthy lifestyle habits and optimal pharmacological therapy. In this regard, adherence can be improved via the polypill and simplified therapeutic regimens. Multidisciplinary cardiac rehabilitation programs decrease mortality and hospitalizations and improve quality of life in patients with chronic coronary syndromes but patients are much less often referred to cardiac rehabilitation after NSTEACS than after STEACS. The guidelines stress the need to increase the referral rate to these programs (upgrading their recommendation to I A) and to strive for gender equity, which is still insufficient Spain. The role of nursing takes center stage in improving communication, cost-effectiveness, and therapeutic adherence to all recommendations.1 In Spain, the creation of new cardiac rehabilitation units and the use of telemedicine are essential to boost the implementation of the recommendations on the secondary prevention of ACS.
Another novel aspect is the reference to cardiovascular risk associated with environmental pollution, not only air, but also acoustic. Finally, annual flu vaccination is recommended (I B), particularly in the elderly.
The most prominent developments in long-term drug therapy focus on lipid-lowering drugs. In line with the guidelines on the management of dyslipidemia, the therapeutic targets for low-density lipoprotein-cholesterol (LDL-C) have been increased: a 50% reduction (baseline values between 70 and 135mg/dL) and LDL-C <55mg/dL.16,17 In addition, the guidelines emphasize that these targets must be achieved as soon as possible (4-6 weeks); if they are not achieved with maximum tolerated doses of statins, ezetimibe (I B) should be added and, if they are not achieved with maximum tolerated doses of both, a PCSK9 inhibitor is advised as well (I B). PSCK9 inhibitors clearly reduce cardiovascular events and are well tolerated, but their implementation in clinical practice is limited by their high cost. In patients with event recurrence before 2 years of statin therapy, a more aggressive target may be considered (LDL-C <40mg/dL) (IIb B).
Long-term beta-blocker therapy has not been modified and the indications of the previous guidelines are maintained.1 The authors point out that, pending results from ongoing studies, there is a lack of clinical trial evidence establishing the need for beta-blocker therapy and its duration in patients without systolic dysfunction.18
The indication for angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers (ACEIs/ARBs) reappears in patients with renal failure (except when contraindicated due to severe renal failure or hyperkalemia). Mineralocorticoid receptor antagonists are indicated in patients with heart failure and an ejection fraction <40% (35% in previous guidelines). A novelty is the indication for sacubitril-valsartan, replacing ACEIs/ARBs in patients with symptomatic heart failure and ejection fraction ≤ 35% despite optimized treatment.
QUALITY INDICATORSThe present guidelines define quality indicators in a more comprehensive and elaborate way than in the previous guidelines. Indicators are shown for each of the following aspects: center organization, invasive strategy, in-hospital risk assessment, antithrombotic therapy during hospitalization, secondary prevention treatments, patient satisfaction, and 30-day mortality. In Spain, the most difficult indicators to achieve would concern: a) center organization: implementation of the 0-1 hour/0-2 hour hs-cTn T/I algorithms; few emergency departments have an organizational level permitting 2 troponin measurements and their results to be obtained within 1 hour; b) an invasive strategy within 24hours in NSTEACS patients with some high-risk characteristics; this indicator requires well-equipped catheterization laboratories and a correct evaluation of the patient to distinguish high-risk type 1 NSTEACS from type 2 NSTEACS or nonischemic myocardial injury and to not make unnecessary indications for early catheterization; this recommendation may be very difficult to apply in Spain because it will often involve patient transfer to another center with catheterization facilities; and c) evaluation of patient satisfaction and analysis of 30-day mortality: few Spanish centers perform these types of measurement.
MANAGEMENT STRATEGYWhat to do and what not to doThis is one of the most important sections of the guidelines because it summarizes the class I (recommended) and III (not recommended) indications in tables. Notably, of a total of 78 recommendations with theoretically clear evidence supporting what to do (70) and what not to do (8), only 32% have level of evidence A vs 38.6% and 29.4% with levels B and C. This highlights the gaps in evidence needing to be resolved regarding multiple aspects in the management of NSTEACS.
Key messagesThe key messages section provides an excellent summary of the main contributions of the guidelines. It includes 15 key messages; 4 are focused on hs-cTn T/I and 3 on antithrombotic therapy. No key messages are highlighted regarding secondary prevention strategies or quality indicators. Figure 1 schematizes the comprehensive management of patients with NSTEACS according to these messages.
Gaps in evidenceThe guidelines recognize numerous gaps remaining to be resolved through new clinical trials. Among them, it should be noted that the risk stratification of patients has been recommended for decades, so much so that it is assumed that the higher the risk, the more aggressive the required treatment. However, this relationship has never been confirmed. The drug therapy for AF patients undergoing PCI requires expert recommendations based on current scientific evidence, as well as an agreement among the different associations involved in their drafting. Another aspect of special economic interest, as well as for patients, is to know how long treatment with beta-blockers or inhibitors of the renin-angiotensin-aldosterone system should be maintained in patients with normal ventricular function and with no other indications for these treatments.
CONFLICTS OF INTERESTThe authors declare no conflicts related to this article.
SEC Working Group for the 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Antonia Sambola (coordinator), Pablo Avanzas (coordinator), Rut Andrea, Albert Ariza, Gemma Berga, Belén Cid, Esteban López de Sa, Manuel Martínez-Sellés, Raúl Moreno, Soledad Ojeda, and Juan Sanchis.
Expert reviewers for the 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Ana Huelmos, Pilar Jiménez Quevedo, Miriam Juárez, Roberto Martín Asenjo, Mila Pedreira, Oriol Rodríguez Leor, Inmaculada Roldán, Rafael Romaguera, and Ana Viana Tejedor.
SEC Guidelines Committee: Pablo Avanzas, Gemma Berga Congost, Araceli Boraita, Héctor Bueno, David Calvo, Raquel Campuzano, Victoria Delgado, Laura Dos, Ignacio Ferreira-González, Juan José Gómez Doblas, Domingo Pascual Figal, Antonia Sambola Ayala, Ana Viana Tejedor, José Luis Ferreiro (copresident), and Fernando Alfonso (copresident).