This article analyzes the European Society of Cardiology (ESC) update1 of the last guidelines on ventricular arrhythmias and sudden cardiac death (SCD), published in 2006.2 Emphasis is placed more on the prevention of SCD than on treatment of ventricular arrhythmias, and there are several comments of practical interest for the management of these patients. Adherence to these guidelines is considered a quality indicator.3
DEFINITIONS, EPIDEMIOLOGY, AND FUTURE PERSPECTIVESAutopsy and Molecular Autopsy of Sudden Death VictimsIn the present guidelines,1 autopsy is given a I-C recommendation to determine the cause of the unexpected death. Histology of the heart and toxicological evaluation of the blood and other fluids is a Class I recommendation. Postmortem genetic assessment is considered a Class IIa recommendation when cardiac channelopathy is suspected. However, the guidelines advise against the use of large panels of genes in SCD assessment and recommend targeted genetic study.
TREATMENT OF VENTRICULAR ARRHYTHMIASPharmacological TherapyBeta-blockersBeta-blockers have shown a beneficial effect in patients with ventricular dysfunction, with or without associated heart failure. However, the present guidelines mention a registry4 of acute myocardial infarction (AMI) patients showing an unfavorable effect in those with 2 or more of the following factors: age older than 70 years, heart rate >110 bpm, and blood pressure <110mmHg. This deleterious effect seems restricted to very ill patients with severe ventricular dysfunction and has not been confirmed in other studies; therefore, it should be viewed with caution.
AmiodaroneIn the SCD-HeFT5 study, amiodarone had a neutral effect on mortality in patients with ventricular dysfunction (left-ventricular ejection fraction [LVEF] < 35%). The drug can be used to reduce the arrhythmia burden in patients with an indication for defibrillator placement.
Sotalol/d-sotalolThe importance of sotalol has declined over the years. It can be used in patients with ischemic heart disease and no heart failure. D-sotalol, a drug that is not marketed in Spain, has been associated with increased mortality in patients with ventricular dysfunction following AMI.
Automatic Implantable Cardioverter DefibrillatorCardioverter defibrillator implantation is the most effective option for both primary and secondary prevention of SCD, and its use is growing in our setting.6 Since the 2006 guidelines,2 several long-term studies have confirmed the benefits of implantable cardioverter defibrillators (ICD). Traditionally, it was considered necessary to maintain a waiting period of 40 days from the AMI event to device implantation. An interesting innovation in the approach now allows “prompt” (< 40 days) implantation when specific conditions are met, such as pre-existing LVEF dysfunction, incomplete revascularization, and the presence of nonsustained ventricular tachycardia more than 48hours after the event. This is one of the most controversial aspects of these new guidelines, as it is not based on firm evidence and there are alternative options, such as wearable defibrillator vests (Table 1).
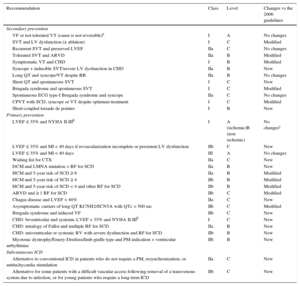
Defibrillator Indications
| Recommendation | Class | Level | Changes vs the 2006 guidelines |
|---|---|---|---|
| Secondary prevention | |||
| VF or not tolerated VT (cause is not reversible)a | I | A | No changes |
| SVT and LV dysfunction (± ablation) | I | C | Modified |
| Recurrent SVT and preserved LVEF | IIa | C | No changes |
| Tolerated SVT and ARVD | IIa | B | Modified |
| Symptomatic VT and CHD | I | B | Modified |
| Syncope + inducible SVT/severe LV dysfunction in CHD | IIa | B | New |
| Long QT and syncope/VT despite BB | IIa | B | No changes |
| Short QT and spontaneous SVT | I | C | New |
| Brugada syndrome and spontaneous SVT | I | C | Modified |
| Spontaneous ECG type-I Brugada syndrome and syncope | IIa | C | No changes |
| CPVT with SCD, syncope or VT despite optimum treatment | I | C | Modified |
| Short-coupled torsade de pointes | I | B | New |
| Primary prevention | |||
| LVEF ≤ 35% and NYHA II-IIIb | I | A (ischemic)B (non ischemic) | No changesc |
| LVEF ≤ 35% and MI < 40 days if revascularization incomplete or persistent LV dysfunction | IIb | C | New |
| LVEF ≤ 35% and MI < 40 days | III | A | No changes |
| Waiting list for CTX | IIa | C | New |
| DCM and LMNA mutation + RF for SCD | IIa | B | New |
| HCM and 5-year risk of SCD ≥ 6 | IIa | B | Modified |
| HCM and 5-year risk of SCD ≥ 4 | IIb | B | Modified |
| HCM and 5-year risk of SCD < 4 and other RF for SCD | IIb | B | Modified |
| ARVD and ≥ 1 RF for SCD | IIb | C | Modified |
| Chagas disease and LVEF < 40% | IIa | C | New |
| Asymptomatic carriers of long QT KCNH2/SCN5A with QTc > 500 ms | IIb | C | Modified |
| Brugada syndrome and induced VF | IIb | C | New |
| CHD: biventricular and systemic LVEF < 35% and NYHA II-IIIb | I | C | New |
| CHD: tetralogy of Fallot and multiple RF for SCD | IIa | B | New |
| CHD: univentricular or systemic RV with severe dysfunction and RF for SCD | IIb | B | New |
| Myotonic dystrophy/Emery-Dreifuss/limb-girdle type and PM indication + ventricular arrhythmias | IIb | B | New |
| Subcutaneous ICD | |||
| Alternative to conventional ICD in patients who do not require a PM, resynchronization, or antitachycardia stimulation | IIa | C | New |
| Alternative for some patients with a difficult vascular access following removal of a transvenous system due to infection, or for young patients who require a long-term ICD | IIb | C | New |
BB, beta-blockers; CTX, cardiac transplantation; ECG, electrocardiography; LMNA, laminin A/C mutation; LV, left ventricle; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association functional class; PM, pacemaker; RF, risk factors; RV, right ventricle; SCD, sudden cardiac death; VF, ventricular fibrillation; SVT, sustained ventricular tachycardia.
Includes situations such as dilated cardiomyopathies (DCM, Level A), hypertrophic cardiomyopathy (HCM, Level B), arrhythmogenic RV dysplasia (ARVD, Level C), restrictive cardiomyopathy (RCM, Level C), infiltrative cardiomyopathy (Level C), long QT syndrome (Level B), short QT syndrome (level C), Brugada syndrome (Level C) and catecholaminergic polymorphic VT (CPVT, Level C), congenital heart disease (CHD, Level B), idiopathic VF (Level B), short-coupled torsade de pointes (Level B), valvular disease (Level C), pregnancy (Level C), and pediatric age (Level B), although with different levels of evidence (in parentheses) and even with a lower recommendation grade, as in inflammatory heart disease (Class IIa, Level C), amyloidosis (Class IIa, Level C) and giant cell myocarditis and sarcoidosis (Class IIb, Level C).
The transvenous leads are the most vulnerable components of subcutaneous ICD. A recently developed defibrillator system with subcutaneous electrodes has proven effective in preventing SCD due to ventricular arrhythmias.7 Pending the publication of randomized studies, the initial results are promising (Table 1).
Wearable Cardioverter DefibrillatorExternal cardioverter defibrillators attached to a wearable vest are recommended (IIb-C) for limited use in patients without an ICD indication as a bridge to cardiac transplantation, following an AMI with poor LVEF, and in conditions that can improve with time (eg, myocarditis and peripartum cardiomyopathy). Although there are no prospective studies investigating their effectiveness, registries have reported successful use of these devices in the treatment of ventricular arrhythmias.
Public Access DefibrillationThe guidelines recommend the establishment of external defibrillators (I-B) in busy public places where there is a relatively high possibility of cardiac arrest, such as schools, sports stadiums, and public transport stations. These defibrillators may also be considered for home use (Class IIb recommendation) in families with a high risk of SCD.
Catheter AblationCatheter ablation is considered a Class I, Level B recommendation for the treatment of electrical storm and for patients with ICD delivering recurrent shocks. It is also indicated after a first episode of ventricular tachycardia (VT) in patients with ischemic heart disease (IIa-B). Nonetheless, VT ablation is difficult and the outcome is highly operator-dependent (experience and volume of patients treated). Therefore, prompt transfer to a specialized center is recommended in patients with electrical storm.
Anti-arrhythmic SurgerySurgery for arrhythmias is uncommon and the procedures should be performed in experienced centers. Surgery can be considered in arrhythmias refractory to medical therapy and after failed catheter ablation, particularly if surgical revascularization is planned and the patient has a ventricular aneurysm.
TREATMENT OF VENTRICULAR ARRHYTHMIAS AND PREVENTION OF SUDDEN DEATH IN ISCHEMIC HEART DISEASEFrom the clinical viewpoint, this section is much more coherent and comprehensible than the corresponding one in the previous guidelines. A limited number of innovations have been incorporated related to the following:
- •
Prompt beta-blocker use in AMI
- •
Role of mechanical cardiac assist devices in the acute phase
- •
Role of catheter ablation to treat refractory arrhythmias
- •
Lack of efficacy of ICD in the first weeks following an AMI
- •
Confirmation of the prognostic impact of arrhythmia in the acute phase
- •
Value of programmed electrical stimulation in AMI survivors with preserved LVEF, and in those with reduced LVEF, definition of a group at lower SCD risk when the results are negative.
One important general aspect is the level of evidence of the recommendations: 58% are Level C and only 8% Level A, with 55% Class I and 35% Class IIa or IIb.
One example is the indication for urgent coronary angiography (<2hours) in persistently comatose patients resuscitated from SCD of uncertain etiology (IIa-B). This procedure was performed in stable patients only in one of the articles cited8 and was limited to patients younger than 75 years in another article.9 However, the updated guidelines particularly recommend coronary angiography in hemodynamically unstable patients. Although this Class IIa recommendation could be adopted with reservations, the level of evidence is questionable (1 large, nonrandomized study) and the recommendation might be more appropriately rated Level C, especially in the case of hemodynamic instability.
The guidelines stress the need for prompt, complete revascularization in patients with ventricular arrhythmia or cardiac arrest within an acute coronary syndrome, or at the mere suspicion of these conditions and in the absence of other possible causes.
THERAPY FOR PATIENTS WITH LEFT VENTRICULAR DYSFUNCTION WITH OR WITHOUT HEART FAILUREThe writing has been simplified in this section and is more precise. The LVEF range of 30% to 40% and the differences between ischemic and nonischemic cardiomyopathy have been eliminated, and the same recommendations have been established for the 2 substrates (LVEF <35%). The new guidelines maintain the recommendations in the heart failure guidelines.10
No recommendations are provided on the use of ICD for SCD prevention in patients with milder degrees of left ventricular dysfunction (LVEF > 35%) because of the absence of controlled studies in this line. For the first time, there is an indication for ICD placement in patients on the waiting list for a heart transplant, even though many of them do not meet the condition of ≥ 1 year life expectancy, either because of their high risk of death in itself, or because they have been on the transplant waiting list for > 1 year.
Another aspect under discussion is the benefit of resynchronization therapy in patients without left bundle branch block (LBBB), especially if the QRS complex duration is <150ms. This recommendation has a Class IIb, Level B rating, the same as in the resynchronization guidelines.11
The recommendations in the previous resynchronization guidelines11 have been adopted for patients with atrial fibrillation, and a Class IIa recommendation has been established for patients in New York Heart Association (NYHA) functional class III or IV, providing that near 100% biventricular pacing can be guaranteed.
Finally, catheter ablation of ventricular extrasystoles (VEs) is indicated for patients with left ventricular dysfunction. It is considered a Class IIa recommendation if VEs are frequent, especially at an overall density greater than 24%. Other variables that could be of help, such as VE morphology, have not been taken into consideration. Some of these factors might point to an idiopathic origin of VEs, which would denote a higher probability of success with ablation treatment, or to the presence of various morphologies, which would indicate the opposite.
CARDIOMYOPATHIESAs in the previous version,2 the updated guidelines focus on the management of ventricular arrhythmias and SCD prevention in dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy, and arrhythmogenic right ventricular dysplasia.
Dilated CardiomyopathyMagnetic resonance imaging (MRI) is not recommended for stratifying SCD risk in patients with DCM. One innovation in this section is the use of electrophysiologic assessment for prognostic stratification of DCM (IIb-B). This practice is unusual because of the low sensitivity of the test, and the recommendation is controversial. In contrast to the previous guidelines, the update recommends (I-B) coronary angiography in stable DCM patients with an intermediate risk of coronary disease and new-onset ventricular arrhythmia. ICD implantation is recommended for patients with poor hemodynamic tolerance of ventricular arrhythmias, regardless of the LVEF (I-A), and for patients with DCM and LVEF <35% in NYHA class II or III (I-B). The previous guidelines recommended ICD implantation in DCM patients with syncope and significant left ventricular dysfunction, but in the update, management of DCM patients with syncope has been eliminated. Removal of this recommendation may generate confusion. The recommendation for an ICD in patients in NYHA class I has also disappeared.
The main therapeutic innovation in the update is the recommendation for ICD implantation (IIa-B) in patients with DCM and clinical risk factors (nonsustained VT, male sex, and LVEF <45%) after confirmation of a causative mutation in the laminin gene.
Identification and treatment of proarrhythmogenic risk factors is stressed (I-B). Amiodarone is recommended in patients with recurrent appropriate shocks despite optimal device programming (IIa-C), but should not be used in patients with asymptomatic episodes of nonsustained VT (III-A). Dronedarone and sodium channel blockers are contraindicated because of their proarrhythmic risk. The role of catheter ablation for VT in DCM patients is covered in a specific section: The recommendation for ablation in bundle branch re-entry VT is maintained (I-B), whereas ablation is limited to patients refractory to medical therapy in VT secondary to other mechanisms (IIb-C). The grading of the recommendation is lower than that of a previous consensus statement12 (IIa-B).
Hypertrophic CardiomyopathyIn patients with hypertrophic cardiomyopathy, the current update adopts the risk stratification approach of the 2014 European guidelines.13,14 Risk stratification for SCD is recommended in individuals older than 16 years (I-B) at the time of the initial evaluation and every 1 or 2 years thereafter or when there is a change in the clinical status (I-B). Avoidance of competitive sports is recommended (I-C). In contrast to the previous guidelines, the update advises against electrophysiologic evaluation for prognostic stratification (III-C). ICD implantation may be considered in secondary prevention (I-B) and in primary prevention for patients with an estimated 5-year risk of SCD >6% (IIa-B) or between 4% and 6% (IIb-B). ICD placement is also recommended (IIb-B) in patients with an estimated 5-year risk < 4% when they have other clinical features of prognostic value.
In pediatric patients, the European guideline recommendations for ICD placement have been maintained: ICD can be used in secondary prevention and in primary prevention when the patient has 2 or more of the major risk factors for SCD in children. In patients with a single risk factor, the indication for an ICD can be evaluated on an individual basis.
Arrhythmogenic Right Ventricular DysplasiaSome general recommendations are incorporated in this section, such as avoidance of competitive sports (I-C), beta-blocker therapy in symptomatic patients with ventricular extrasystoles or runs of nonsustained VT (I-C), and amiodarone therapy in patients who are intolerant of or have contraindications to beta blockers (IIa-C). ICD implantation is recommended in secondary prevention for patients with hemodynamically poorly tolerated arrhythmias (I-C), those with hemodynamically well-tolerated VT (IIa-C), and those with syncope. ICD placement can be considered for primary prevention in patients with risk factors (recommendation rated IIb-C vs IIa-C in the previous guidelines). Finally, catheter ablation should be considered in patients with frequent episodes of VT, with the aim of improving the symptoms or reducing the number of ICD shocks (IIa-B).
Other CardiomyopathiesRecommendations have been established for several types of cardiomyopathies that were not present in the previous guidelines. ICD implantation should be considered in patients with cardiac amyloidosis and ventricular arrhythmia causing hemodynamic repercussions (IIa-C) and in those with restrictive cardiomyopathy (I-C) who are expected to survive > 1 year. Finally, Chagas disease is addressed for the first time, with a recommendation for ICD implantation in patients with Chagas cardiomyopathy and LVEF < 40% (IIa-C).
INHERITED PRIMARY ARRHYTHMIA SYNDROMESGeneral AspectsUnfortunately, evidence remains scarce in genetic arrhythmia syndromes, and 86% (31 of 36) of the related recommendations are based on Level C evidence. The only recommendations with Level B evidence are those for long QT syndrome, which has been evaluated in registries including large numbers of patients and lengthy follow-up periods.
One innovation in the section on inherited primary arrhythmia syndromes is that diagnostic criteria have been defined for each of the diseases included (with the exception of early repolarization syndrome). These criteria were proposed in a previous expert consensus statement,15 but certain small modifications have been made (Table 2).
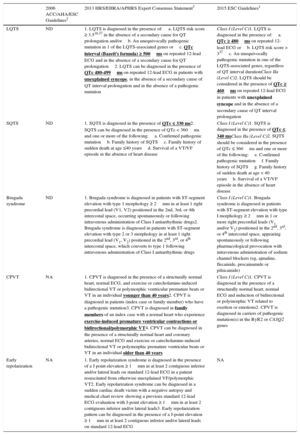
Recommendations for the Diagnosis of Inherited Primary Arrhythmia Syndromes
| 2006 ACC/AHA/ESC Guidelines2 | 2013 HRS/EHRA/APHRS Expert Consensus Statement3 | 2015 ESC Guidelines1 | |
|---|---|---|---|
| LQTS | ND | 1. LQTS is diagnosed in the presence of:a. LQTS risk score ≥ 3.516,37 in the absence of a secondary cause for QT prolongation and/orb. An unequivocally pathogenic mutation in 1 of the LQTS-associated genes orc. QTc interval (Bazett's formula) ≥ 500ms on repeated 12-lead ECG and in the absence of a secondary cause for QT prolongation2. LQTS can be diagnosed in the presence of QTc 480-499ms on repeated 12-lead ECG in patients with unexplained syncope, in the absence of a secondary cause of QT interval prolongation and in the absence of a pathogenic mutation | Class I (Level C)1. LQTS is diagnosed in the presence of:a. QTc ≥ 480ms on repeated 12-lead ECG orb. LQTS risk score > 337c. An unequivocally pathogenic mutation in one of the LQTS-associated genes, regardless of QT interval durationClass IIa (Level C)2. LQTS should be considered in the presence of QTc ≥ 460ms on repeated 12-lead ECG in patients with unexplained syncope and in the absence of a secondary cause of QT interval prolongation |
| SQTS | ND | 1. SQTS is diagnosed in the presence of QTc ≤ 330 ms2. SQTS can be diagnosed in the presence of QTc < 360ms and one or more of the following:a. Confirmed pathogenic mutationb. Family history of SQTSc. Family history of sudden death at age ≤40 yearsd. Survival of a VT/VF episode in the absence of heart disease | Class I (Level C)1. SQTS is diagnosed in the presence of QTc ≤ 340 msClass IIa (Level C)2. SQTS should be considered in the presence of QTc ≤ 360ms and one or more of the following:e. Confirmed pathogenic mutationf. Family history of SQTSg. Family history of sudden death at age < 40 yearsh. Survival of a VT/VF episode in the absence of heart disease |
| Brugada syndrome | ND | 1. Brugada syndrome is diagnosed in patients with ST-segment elevation with type 1 morphology ≥ 2mm in at least 1 right precordial lead (V1, V2) positioned in the 2nd, 3rd, or 4th intercostal space, occurring spontaneously or following intravenous administration of Class I antiarrhythmic drugs2. Brugada syndrome is diagnosed in patients with ST-segment elevation with type 2 or 3 morphology in at least 1 right precordial lead (V1, V2) positioned in the 2nd, 3rd, or 4th intercostal space, which converts to type 1 following intravenous administration of Class I antiarrhythmic drugs | Class I (Level C)1. Brugada syndrome is diagnosed in patients with ST-segment elevation with type I morphology ≥ 2mm in 1 or more right precordial leads (V1 and/or V2) positioned in the 2nd, 3rd, or 4th intercostal space, appearing spontaneously or following pharmacological provocation with intravenous administration of sodium channel blockers (eg, ajmaline, flecainide, procainamide or pilsicainide) |
| CPVT | NA | 1. CPVT is diagnosed in the presence of a structurally normal heart, normal ECG, and exercise or catecholamine-induced bidirectional VT or polymorphic ventricular premature beats or VT in an individual younger than 40 years2. CPVT is diagnosed in patients (index case or family member) who have a pathogenic mutation3. CPVT is diagnosed in family members of an index case with a normal heart who experience exercise-induced premature ventricular contractions or bidirectional/polymorphic VT4. CPVT can be diagnosed in the presence of a structurally normal heart and coronary arteries, normal ECG and exercise or catecholamine-induced bidirectional VT or polymorphic premature ventricular beats or VT in an individual older than 40 years | Class I (Level C)1. CPVT is diagnosed in the presence of a structurally normal heart, normal ECG and induction of bidirectional or polymorphic VT related to exertion or emotions2. CPVT is diagnosed in carriers of pathogenic mutation(s) in the RyR2 or CASQ2 genes |
| Early repolarization | NA | 1. Early repolarization syndrome is diagnosed in the presence of a J point elevation ≥ 1mm in at least 2 contiguous inferior and/or lateral leads on standard 12-lead ECG in a patient resuscitated from otherwise unexplained VF/polymorphic VT2. Early repolarization syndrome can be diagnosed in a sudden cardiac death victim with a negative autopsy and medical chart review showing a previous standard 12-lead ECG evaluation with J-point elevation ≥ 1mm in at least 2 contiguous inferior and/or lateral leads3. Early repolarization pattern can be diagnosed in the presence of a J-point elevation ≥ 1mm in at least 2 contiguous inferior and/or lateral leads on standard 12-lead ECG | NA |
CPVT, catecholaminergic polymorphic ventricular tachycardia; ECG, electrocardiography; LQTS, long QT syndrome; LVEF, left ventricular ejection fraction; SQTS, short QT syndrome; VF, ventricular fibrillation; VT, ventricular tachycardia
*In bold and underlined, the main differences in the diagnosis of inherited primary arrhythmia syndromes.
Programmed ventricular stimulation is not recommended in patients with long QT syndrome, short QT syndrome, or catecholaminergic polymorphic VT. However, analysis of sustained ventricular arrhythmia inducibility in Brugada syndrome is still considered optional (IIb-C), despite the huge controversy generated by the negative outcome in the PRELUDE study.16 ICD implantation remains the therapy of choice for survivors of cardiac arrest secondary to polymorphic VT or ventricular fibrillation (VF). Nonetheless, evidence is scarce supporting the ICD indication for primary prevention in this group of diseases, and few related recommendations are included.
It would be of value to establish recommendations for screening of family members in primary arrhythmias.
Long QT SyndromeImportant New AspectsThe proposed diagnostic criteria for long QT syndrome in the 2013 HRS/EHRA/APHRS expert consensus document have been modified in the current guidelines. The panel considered that the QTc thresholds of > 500ms in asymptomatic patients and > 480ms in patients with unexplained syncope were too conservative; hence, the QTc thresholds have been lowered to ≥ 480 and ≥ 460ms, respectively. One innovation is the new recommendation for ICD implantation in primary prevention for certain high-risk subgroups with a KCNH2 or SCN5A mutation and QTc >500ms (IIb-C).
Aspects that Have not Been CoveredAlthough there is evidence supporting the use of stress testing in the diagnosis of long QT syndrome, there is no mention of the test for this purpose. Nor is there any discussion regarding the advisability of DNA analysis in long QT syndrome index cases.
Short QT SyndromeControversial AspectsFew cases of short QT syndrome have been reported since the first description of this channelopathy, and the related diagnostic criteria are still a subject of debate. The proposed cut-offs have empirical value: Short QT syndrome is diagnosed in the presence of QTc ≤340ms in asymptomatic patients and ≤360ms in those with a pathogenic mutation, or a family history of short QT syndrome, or SCD in a family member younger than 40 years, or survival of a sustained ventricular arrhythmia event in the absence of heart disease. Furthermore, the recommended therapy (sotalol or quinidine) is based on small series. Therefore these recommendations should be viewed with caution until new evidence is available.
Brugada SyndromeImportant New AspectsThe diagnosis of Brugada syndrome is based on an ST segment elevation of ≥2mm in at least 1 right precordial lead, positioned in the second, third, or fourth intercostal space, seen at baseline or following administration of sodium channel blockers. The promising role of epicardial ablation over the right ventricular outflow tract is mentioned for patients with recurrent arrhythmia episodes.
Controversial aspectsRisk stratification remains controversial in Brugada syndrome patients, and there are no substantial innovations in this update. The recommendation for ICD implantation has not changed in patients who develop VF during programmed ventricular stimulation (IIb-C).
Catecholaminergic Polymorphic Ventricular TachycardiaImportant New AspectsAn indication for flecainide combined with beta-blockers has appeared as a therapeutic recommendation in patients with an ICD and recurrent ventricular arrhythmias. Left cardiac sympathetic denervation, another new recommendation, is proposed for controlling recurrent symptoms or arrhythmias in patients previously treated with beta-blockers and those who are intolerant to these drugs.
Early Repolarization SyndromeImportant New AspectsThe authors have chosen not to propose recommendations for the management of early repolarization syndrome because of the many uncertainties regarding this condition. It is suggested that the diagnosis should be made only in patients with an early repolarization pattern in the inferior or lateral leads and an episode of idiopathic VF/polymorphic VT.
PEDIATRIC ARRHYTHMIAS AND CONGENITAL HEART DISEASEIn this section, ventricular arrhythmias are divided into the following: 1) arrhythmias in structurally normal hearts, 2) arrhythmias in patients with congenital heart disease, and 3) indications for ICD implantation in pediatric patients.
ICD placement is recommended in children with congenital heart disease resuscitated from SCD and in those with symptomatic VT. ICD therapy is also recommended in adult patients in NYHA class II or III with LVEF <35% and heart failure. Catheter ablation is recommended in patients with recurrent monomorphic tachycardia or multiple appropriate ICD shocks. ICD placement can be considered in patients with syncope in the presence of either ventricular dysfunction or inducible arrhythmia, and in those with tetralogy of Fallot and associated SCD risk factors, such as ventricular dysfunction, nonsustained VT, a very wide QRS, or inducible arrhythmia. Ablation is also proposed for patients with an ICD who experience recurrent monomorphic VT. The authors mention the possibility of using electrophysiologic stimulation for SCD risk stratification in patients with tetralogy of Fallot or nonsustained VT, but there is no formal indication in this line. The use of anti-arrhythmic therapy or catheter ablation is not recommended for asymptomatic infrequent extrasystoles in patients with a preserved LVEF. Programmed stimulation should not be used in patients with congenital heart disease and no other risk factors.
VENTRICULAR TACHYCARDIAS AND VENTRICULAR FIBRILLATION IN STRUCTURALLY NORMAL HEARTSOutflow Tract Ventricular TachycardiaWith regard to idiopathic right ventricular outflow tract tachycardia, the recommendation for ablation has been raised from I-C to I-B in patients refractory to, intolerant of, or refusing anti-arrhythmic therapy. The recommendation also includes patients with a depressed LVEF attributable to a high burden of ventricular extrasystoles.
Another important innovation related to the management of arrhythmogenic substrates of the outflow tract is that the type of management will differ according to the presumed origin. Catheter ablation is recommended as first-line therapy (I-B) if the suspected origin is the right ventricular outflow tract, whereas therapy with sodium channel blockers would be the first approach if the suspected origin is the left ventricle (LV) (aortic cusp, LV outflow tract, LV outflow tract epicardium, or pulmonary artery) (I-C). The guidelines mention that ablation procedures are more complex in suspected left sources, as combined access approaches are often required. Hence, left ventricular outflow tract ablation should only be done in symptomatic patients who have failed at least 1 class I-C drug and in centers with considerable ablation experience (II-B).
A limitation of this section is that there are no recommendations for patients with a specific suspicion of tachycardiomyopathy due to VT/VE of the LV outflow tract.
Idiopathic Ventricular Tachycardias Of Other OriginsA new subsection has been created regarding the management of idiopathic LV and papillary muscle tachycardia, and mitral and tricuspid annular tachycardia. Catheter ablation is considered the first-line treatment (I-B) in patients with symptomatic idiopathic LV tachycardias (fascicular, bundle branch, Purkinje system, or interfascicular), whereas drug therapy with calcium channel blockers, beta-blockers or class I-C drugs is recommended as a second-line option (I-C). As to papillary muscle and annular tachycardias, the guidelines have issued a new II-B indication for ablation, whereas drug therapy would be the initial treatment of choice (I-A).
Idiopathic Ventricular Fibrillation and Short-coupled Torsade de PointesThe document recognizes that the diagnosis of idiopathic VF is currently by exclusion, although our knowledge of this condition may advance in the future. ICD placement remains a treatment standard (I-B) in patients without structural heart disease who have survived VF, and for the first time, catheter ablation is granted this same level of evidence for treating VE clearly proven to trigger VT/VF or repeated ICD shocks.
There is a new specific section for patients with short-coupled torsade de pointes VT. ICD placement is recommended for the initial management (I-B), with later use of verapamil and ablation of the triggers to prevent electrical storm or recurrent shocks.
INFLAMMATORY, RHEUMATIC, AND VALVULAR HEART DISEASESMyocarditisAlthough this section contains numerous changes, all the recommendations remain at evidence level C. There is 1 new reco-mmendation (Class IIb) that includes both persistent myocardial inflammatory infiltrates on biopsy and abnormal fibrosis on MRI as risk factors for SCD, thus supporting ICD placement. Ventricular arrhythmias can develop in 2 different scenarios:
- •
Acute myocarditis with refractory VT and severe heart failure is associated with a very poor short-term prognosis in patients with giant cell myocarditis. However, in survivors of the acute phase, the later course is relatively favorable. This life-threatening presentation justifies inclusion of a new recommendation (Class I) to refer these patients to specialized centers.
- •
Myocarditis leading to inflammatory cardiomyopathy has a risk profile for SCD similar to that of dilated cardiomyopathy.
As was mentioned above, these new guidelines include the use of wearable ICD, such as the LifeVest 4000 (ZOLL Lifecor Corp., United States), as bridge therapy (Class IIa).
Endocarditis, rheumatic heart disease, and pericarditisThe discussion of these 3 conditions has been notably reduced, as is reasonable considering that they are rare causes of SCD and usually not due to ventricular arrhythmias, but instead to atrioventricular block (AVB) or hemodynamic changes.
SarcoidosisBecause of the high recurrence rate of VT in cardiac sarcoidosis, ICD implantation has a Class IIa recommendation after the acute episode and Class IIb when the condition presents with cardiac arrest or poorly tolerated VT. Very few patients have sarcoidosis in Spain; the incidence is 1 to 4 per 100 000 inhabitants, a lower value than in the rest of Europe (around 15 per 100 000).
Valvular Heart DiseaseThis section incorporates 3 new recommendations: ICD implantation has recognized benefits and is recommended in patients with general indications for primary or secondary prevention after surgery (Class I), there is a need to rule out bundle branch re-entry VT in patients who develop VT following surgery (Class IIa), and surgical treatment is recommended in patients with acute aortic regurgitation due to endocarditis associated with VT (Class I).
ARRHYTHMIC RISK IN SELECTED POPULATIONSPsychiatric PatientsThe section on psychiatric patients has grown disproportionately, without providing innovations. The most important causative mechanism of SCD in these patients seems to be QT interval prolongation, which is associated with the development of torsade de pointes VT. Three (quite evident) Class I recommendations are offered: 1) dosage adjustment or discontinuation of antipsychotic drug therapy if the QTc reaches a length of more than 500ms or increases more than 60ms from the baseline value; 2) monitoring of plasma potassium concentration to avoid hypokalemia, and 3) avoidance of simultaneous use of more than 1 drug prolonging the QT interval. It is surprising that the fourth recommendation, electrocardiography (ECG) evaluation to measure the QT interval before initiation of drug therapy has received only a Class IIa grade, and there is no recommendation for a new QT measurement immediately after the first or second dose of the drug.
Neurological PatientsRelative to the 2006 guidelines, there is a new reference to the possibility of SCD in epilepsy. Although the guidelines alert to the need for screening to investigate cardiac diseases that might mimic epilepsy, there are no firm recommendations regarding this important point.
In the section on neuromuscular disorders, arrhythmia risk is reviewed in patients with muscular dystrophies. Annual cardiologic follow-up in this population is a Class I recommendation for asymptomatic patients, and the general recommendations are applied in patients who develop VT/VF or severe left ventricular dysfunction (I-C). The Class IIb recommendation for pacemaker implantation is maintained for any degree of AVB appearing in certain dystrophies (Steinert disease, Kearns-Seyre syndrome, and limb-girdle muscular dystrophy) because of the risk of rapid progression. ICD placement is also recommended in patients with Emery-Dreifuss muscular dystrophy and limb-girdle muscular dystrophy type 1B associated with laminin A or C mutations, especially those with malignant VT.
Pregnant PatientsTogether with the 2 recommendations for pregnant women included in the 2006 guidelines (electrical cardioversion [ECV] for VT and poorly tolerated VF, and beta-blockers for long QT syndrome, both Class I), 2 additional Class I recommendations are offered in the update: use of oral metoprolol, propranolol, or verapamil is allowed for maintenance therapy of idiopathic sustained VT, and ICD implantation is recommended if an indication emerges during pregnancy. However, the authors specify that it may be reasonable to wait in the case of peripartum cardiomyopathy, as the condition remits spontaneously in more than half the patients. There is no reference to the use of a defibrillator vest, although it would fulfill the same functions as in myocarditis.
There is a new classification of antiarrhythmic drugs according to their teratogenic potential, and recommendations are provided for acute intravenous therapy in hemodynamically stable VT (sotalol or procainamide, Class IIa) or unstable VT refractory to ECV (amiodarone, Class IIa). The guidelines stress the contraindication for class I-C agents during pregnancy (Class I): angiotensin converting enzyme inhibitors (ACEI) (teratogenicity), angiotensin II receptor blockers II (ARBs) (fetotoxicity), and renin inhibitors, although there are no data on this last drug in humans.
It is surprising that the recommendation for catheter ablation in drug-refractory and poorly-tolerated VTs is rated Class IIb. Furthermore, a specific recommendation for the “fluoroscopy 0” approach to reduce radiation dose in ablation procedures has not been included.
Obstructive Sleep ApneaAnother innovation is the inclusion and extensive discussion of obstructive sleep apnea syndrome. The guidelines indicate that this condition should be included in the differential diagnosis of bradyarrhythmias (Class IIa) and be considered a risk factor for SCD (Class IIb).
Pro-arrhythmia DrugsThe recommendations for drug-related pro-arrhythmia have been simplified, and the tables in the previous guidelines have been substituted for a link (http://www.crediblemeds.org) to a website from the University of Arizona that lists all the drugs and drug interactions affecting the QT interval. Only 2 recommendations have been established in relation to suspected drug-induced arrhythmia: 1) withdraw the agents after excluding other possible causes (Class I), and 2) despite drug discontinuation, evaluate whether the patient continues to have a substantial risk of VT/VF advising ICD implantation (Class IIa).
Sudden Cardiac Death after Heart TransplantationPlacement of an ICD following heart transplantation may be appropriate in high-risk patients: cardiac arrest, LVEF <35%, severe graft vasculopathy or syncope of unexplained origin.
Sudden Cardiac Death in AthletesThe Class I recommendation advising careful history taking in athletes has been maintained. The update insists on the contradiction of recommending (Class I) echocardiographic or MRI assessment when abnormal ECG findings suggest a heart disease. A Class IIa rating is given to the recommendation for echocardiography and physical examination in both younger and middle-aged individuals before participating in sports (in the previous guidelines, Class IIb).
These guidelines have added (IIa) an evaluation protocol for assessing middle aged/ senior individuals who wish to engage in sports, adapted from the proposal of the European Association of Cardiovascular Prevention and Rehabilitation. Key elements include the level of activity of the sport and the individual's ESC risk score. Clinical history taking, physical examination, and ECG assessment are recommended in those with higher risk. There are obvious cost-effectiveness concerns related to this strategy, but some studies have reported a cost of $199 per athlete, lower than that of the sports equipment.
Finally, these guidelines introduce a Class IIa recommendation advising training of the staff and coaches at sports facilities in cardiopulmonary resuscitation and in proper use of external defibrillators.
Wolff-Parkinson-White SyndromeTwo recommendations are defined for catheter ablation: patients with Wolff-Parkinson-White syndrome resuscitated from cardiac arrest due to atrial fibrillation-related VF (Class IB) and symptomatic patients or those with an effective refractory period of the accessory pathway (ERPAP) ≤240ms (IIa-B). This latter recommendation constitutes a change with respect to the last (2003) guidelines on supraventricular tachycardias from the ACC/AHA/ESC, in which ablation in patients with Wolff-Parkinson-White syndrome was a Class IB recommendation.
The Terminally Ill PatientTwo Class IIa recommendations recognize the following needs: 1) to consider ICD deactivation according to the desires of the patient or family when clinical conditions deteriorate, and 2) before DAI implantation and during follow-up, to determine the patient's wishes regarding end-of-life issues.
CONFLICTS OF INTERESTNone declared.
SEC Working Group for the 2015 ESC Guidelines for the Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Ignacio Fernández-Lozano (coordinator), Josep Brugada (coordinator), Javier Alzueta, Elena Arbelo, Fernando Arribas, Ignacio García-Bolao, José Luis Merino, Juan José Olalla, Joaquín Osca and Aurelio Quesada.
Expert Reviewers for the 2015 ESC Guidelines for the Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Jesús Almendral Garrote, Ignasi Anguera Camos, Ángel Arenal Maiz, Antonio Berruezo Sánchez, David Calvo Cuervo, Ernesto Díaz Infante, David Filgueiras Rama, Arcadio García Alberola, José Guerra Ramos, Javier Jiménez Candil, José Ormaetxe Merodio, Alonso Pedrote Martínez, Julián Pérez-Villacastín Domínguez, Ricardo Ruiz Granell and Luis Tercedor Sánchez.
SEC Guidelines Committee: Manuel Anguita (president), Ángel Cequier, Fernando Alfonso, Lina Badimón, José A. Barrabés, Ignacio Fernández Lozano, José Juan Gómez de Diego, Luis Rodríguez Padial, José Alberto San Román, Pedro-Luis Sánchez, Juan Sanchís and Alessandro Sionis.
SEC Working Group for the 2015 ESC Guidelines for the Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death, Expert Reviewers for the 2015 ESC Guidelines for the Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death, and the SEC Guidelines Committee.