The Spanish Society of Cardiology (SEC) endorses the clinical practice guidelines (CPGs) of the European Society of Cardiology (ESC). In addition, to improve their dissemination and facilitate their implementation, the CPGs of the ESC are translated into Spanish and published in the online version of Revista Española de Cardiología. These publications are accompanied by an editorial authored by a group of Spanish experts that highlights the most important contributions of each CPG, detailing their changes and developments with respect to previous guidelines and discussing their most controversial aspects and possible limitations. Finally, the recommendations are evaluated and adapted according to the context of the Spanish health care system and clinical practice.
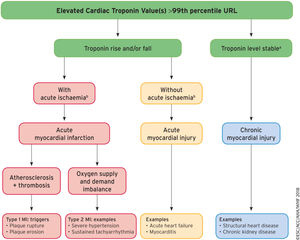
The consensus document of the ESC1 on the Fourth Universal Definition of Myocardial Infarction (4UDMI) is an update of the document published in 2012 and is required reading for both cardiologists and all health care professionals involved in the diagnosis of myocardial infarction or in the interpretation of a finding of elevated cardiac troponins (cTn). The importance of this document lies not only in the changes made, but in the opportunity provided to more clearly explain the difference between the terms “myocardial infarction” (MI) and “myocardial injury” and the different types of MI. This is due to the much more educational orientation conferred to the document by the authors, who illustrate the logical clinical sequence that can be followed to differentiate MI and myocardial injury (Figure 1).
A model for interpreting myocardial injury. Ischemic thresholds vary substantially in relation to the magnitude of the stressor and the extent of underlying cardiac disease. MI, myocardial infarction; URL, upper reference limit. Reproduced with permission of Thygesen et al.1 Translated and reprinted by permission of Oxford University Press on behalf of the European Society of Cardiology.
aStable denotes ≤ 20% variation of troponin values in the appropriate clinical context.
bIschemia denotes signs and/or symptoms of clinical myocardial ischemia.
The consensus document also differentiates between MI and the myocardial injury caused by cardiac and noncardiac procedures and considers new aspects in the analysis of electrocardiography (ECG) (such as electrical memory) and of cardiac imaging (such as the role of magnetic resonance imaging [MRI] and coronary computed tomography angiography [CCTA]). Finally, specific sections are added on takotsubo syndrome, MI with nonobstructive coronary arteries (MINOCA), atrial fibrillation, and chronic kidney disease.
CLINICAL DEFINITION OF MYOCARDIAL INFARCTION AND ANATOMOPATHOLOGICAL CHARACTERISTICS OF MYOCARDIAL ISCHEMIA AND INFARCTIONThe clinical definition of MI denotes the presence of acute myocardial injury detected by abnormal cardiac biomarkers in the setting of evidence of acute myocardial ischemia. The new consensus document does not make significant changes regarding the anatomopathological alterations caused by ischemia.
BIOMARKER DETECTION OF MYOCARDIAL INJURY AND MYOCARDIAL INFARCTIONIncreases in cardiac troponin I (cTnI) have not been reported after damage to noncardiac tissues; however, recent studies show that skeletal muscle lesions can elevate cardiac troponin T (cTnT) levels. Nonetheless, there is sufficient evidence to consider both biomarkers to be the most suitable markers of myocardial injury, and the habitual clinical evaluation is recommended of high-sensitivity cardiac troponin (hs-cTn).2,3 A cTn value higher than the 99th percentile of the normal reference population, which constitutes the upper reference limit (URL), is established as a diagnostic criterion for myocardial injury. The damage is considered acute if there are changes (an increase or decrease) in the cTn levels and chronic if they remain stable. Nonetheless, the absence of significant changes in a serial determination of cTn does not rule out acute myocardial injury if the clinical context is compatible.
Importantly, a cTn elevation can be caused by different clinical situations that can coexist in a single patient. Accordingly, MI is defined by the presence of injury caused by myocardial ischemia.
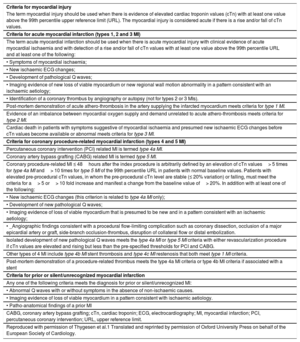
CLINICAL PRESENTATIONS AND CLASSIFICATION OF MYOCARDIAL INFARCTIONOne of the main novelties of the consensus document is the clear differentiation between myocardial injury and MI. The diagnosis of MI requires, in addition to myocardial injury, evidence of myocardial ischemia, in the form of symptoms, electrocardiographic changes, imaging findings, or identification of coronary thrombi by angiography or autopsy (Table 1).
Universal Definitions of Myocardial Injury and Myocardial Infarction
| Criteria for myocardial injury |
| The term myocardial injury should be used when there is evidence of elevated cardiac troponin values (cTn) with at least one value above the 99th percentile upper reference limit (URL). The myocardial injury is considered acute if there is a rise and/or fall of cTn values. |
| Criteria for acute myocardial infarction (types 1, 2 and 3 MI) |
| The term acute myocardial infarction should be used when there is acute myocardial injury with clinical evidence of acute myocardial ischaemia and with detection of a rise and/or fall of cTn values with at least one value above the 99th percentile URL and at least one of the following: |
| • Symptoms of myocardial ischaemia; |
| • New ischaemic ECG changes; |
| • Development of pathological Q waves; |
| • Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischaemic aetiology; |
| • Identification of a coronary thrombus by angiography or autopsy (not for types 2 or 3 MIs). |
| Post-mortem demonstration of acute athero-thrombosis in the artery supplying the infarcted myocardium meets criteria for type 1 MI. |
| Evidence of an imbalance between myocardial oxygen supply and demand unrelated to acute athero-thrombosis meets criteria for type 2 MI. |
| Cardiac death in patients with symptoms suggestive of myocardial ischaemia and presumed new ischaemic ECG changes before cTn values become available or abnormal meets criteria for type 3 MI. |
| Criteria for coronary procedure-related myocardial infarction (types 4 and 5 MI) |
| Percutaneous coronary intervention (PCI) related MI is termed type 4a MI. |
| Coronary artery bypass grafting (CABG) related MI is termed type 5 MI. |
| Coronary procedure-related MI ≤ 48hours after the index procedure is arbitrarily defined by an elevation of cTn values> 5 times for type 4a MI and> 10 times for type 5 MI of the 99th percentile URL in patients with normal baseline values. Patients with elevated pre-procedural cTn values, in whom the pre-procedural cTn level are stable (≤ 20% variation) or falling, must meet the criteria for a> 5 or> 10 fold increase and manifest a change from the baseline value of> 20%. In addition with at least one of the following: |
| • New ischaemic ECG changes (this criterion is related to type 4a MI only); |
| • Development of new pathological Q waves; |
| • Imaging evidence of loss of viable myocardium that is presumed to be new and in a pattern consistent with an ischaemic aetiology; |
| • _Angiographic findings consistent with a procedural flow-limiting complication such as coronary dissection, occlusion of a major epicardial artery or graft, side-branch occlusion-thrombus, disruption of collateral flow or distal embolization. |
| Isolated development of new pathological Q waves meets the type 4a MI or type 5 MI criteria with either revascularization procedure if cTn values are elevated and rising but less than the pre-specified thresholds for PCI and CABG. |
| Other types of 4 MI include type 4b MI stent thrombosis and type 4c MI restenosis that both meet type 1 MI criteria. |
| Post-mortem demonstration of a procedure-related thrombus meets the type 4a MI criteria or type 4b MI criteria if associated with a stent |
| Criteria for prior or silent/unrecognized myocardial infarction |
| Any one of the following criteria meets the diagnosis for prior or silent/unrecognized MI: |
| • Abnormal Q waves with or without symptoms in the absence of non-ischaemic causes. |
| • Imaging evidence of loss of viable myocardium in a pattern consistent with ischaemic aetiology. |
| • Patho-anatomical findings of a prior MI |
| CABG, coronary artery bypass grafting; cTn, cardiac troponin; ECG, electrocardiography; MI, myocardial infarction; PCI, percutaneous coronary intervention; URL, upper reference limit. |
| Reproduced with permission of Thygesen et al.1 Translated and reprinted by permission of Oxford University Press on behalf of the European Society of Cardiology. |
There are no changes regarding the clinical manifestations of MI, and the authors stress the possible presentation of atypical symptoms (palpitations, cardiac arrest) and anginal equivalents (dyspnea, fatigue). The clinical classification of MI is also maintained, although the different clinical contexts are modified.
Type I Myocardial InfarctionThe essential element of type 1 MI is the causal relationship with atherothrombotic coronary disease, triggered by atherosclerotic plaque disruption (in the form of rupture or erosion), and the concept of intraluminal thrombosis is extended to include the possibility of distal embolization and the appearance of hemorrhage within the atherosclerotic plaque.4
Type 2 Myocardial InfarctionType 2 MI is characterized by an imbalance between oxygen supply and demand. Its classification relies on the need to assess the clinical context and the different mechanisms potentially involved in this mismatch. Thus, for example, in patients with stable atherosclerotic coronary disease, tachyarrhythmia or a sudden drop in hemoglobin can cause a type 2 MI due to insufficient blood flow to the ischemic myocardium to meet the myocardial oxygen demand of the stressor. Type 2 MI (and the myocardial injury) has worse prognosis than type 1 MI due to higher noncardiovascular mortality,5,6 although the authors recognize the need for new prospective studies that clarify this relationship.
Finally, the consensus document stresses the differences between the different types of MI, acute myocardial injury (caused by nonischemic acute cardiac events or noncardiac events), and chronic myocardial injury (related to structural heart disease or noncardiac comorbidity) and provides a clinical algorithm that clarifies the concepts and exemplifies each of the categories (Figure).
Type 3 Myocardial InfarctionThe definition of type 3 MI is applied to patients with clinical and electrocardiographic changes indicating myocardial ischemia (which now includes ventricular fibrillation) who die before the cTn can be determined or before elevations are evident. This type differs from sudden cardiac death, which is a larger group that also includes nonischemic cardiac and noncardiac etiologies. Patients initially designated as having type 3 MI should be reclassified as having type 1 MI if an autopsy reveals the presence of a thrombus in a coronary artery.
Coronary Procedure-related Myocardial Injury and Myocardial InfarctionTypes 4 and 5 Myocardial InfarctionIn the consensus document, the definition of type 4 MI–that occurring after a coronary procedure–has not changed with respect to the previous edition.7 This type of MI is arbitrarily defined by an increase in cTn values (> 99th percentile URL) in patients with normal baseline values or an increase in the cTn value> 20% when the level is above the 99th percentile URL but is stable or falling. The high incidence of myocardial injury after percutaneous coronary intervention (PCI) is highlighted, and the authors stress that the preprocedural cTn values have to be stable for diagnosis, as well as the use of cardiac MRI (CMR) with gadolinium to evaluate if there is procedural myocardial injury. In patients with MI or ACS with only a single cTn determination prior to PCI, it is not possible to determine if the cTn elevation is related to the procedure. The prognostic implication of MI/injury despite the use of hs-cTn after the PCI remains controversial.8
Type 4a MI requires a cTn elevation> 5 times the 99th percentile URL in patients with normal baseline values; in patients with a preprocedural cTn elevation whose concentrations are stable (variation ≤ 20%) or decreasing, the cTn increase should be> 20%. In addition, at least 1 criterion of new myocardial ischemia must be present: ECG changes, imaging tests, or angiographic findings showing reduced coronary flow. There is a discrepancy between the text and the summary table regarding other criteria meeting this definition. In the case of the development of new pathological Q waves, it is unclear if this criterion is independent of the hs-cTn or cTn values or requires a cTn increase> 5 times the 99th percentile URL. In the case of the hs-cTn determination, the 5-fold increase proposed in the previous definition is maintained after recent findings.9 Type 4b MI refers to MI caused by stent thrombosis. The same criteria used for type 1 MI are applied, continuing with the temporal classification of this MI subtype recommended by the Academic Research Consortium-2 (ARC-2).10 In type 4c MI, the culprit lesion is a restenosis or complex lesion, and the same criteria apply as for type 1 MI.
The consensus document maintains the previous definition of type 5 MI–that associated with coronary artery bypass grafting–, based on a cTn elevation> 10 times the 99th percentile URL in patients with normal baseline values in the first 48hours after surgery. In patients with previously high and stable or decreasing cTn, the cTn increase should be more than 20%. In both cases, the presence of acute myocardial ischemia is additionally required.11 A marked elevation in cTn values alone indicates a coronary event and has prognostic significance.12 The appearance of new pathological Q waves is diagnostic of type 5 MI if there is cTn elevation, even if it is < 10 times the 99th percentile URL.
Other Definitions of Percutaneous Coronary Intervention- or Coronary Artery Bypass Grafting-related MIARC-210 defines these MIs as any related to any revascularization procedure when there is a postprocedural cTn value ≥ 35 times the 99th percentile URL in patients with a normal baseline cTn value or with elevated preprocedural values in whom the cTn levels are stable or decreasing. In addition, the definition requires an additional criterion indicative of myocardial ischemia. A cTn rise ≥ 70 times the 99th percentile URL is considered a standalone criterion. This definition has been developed in an attempt to minimize the penalization suffered by centers using hs-cTn regarding the incidence of coronary procedure-related complications and MI vs centers that use other types of cTn. The definition to be used from now on remains unclear.
Recurrent Acute Infarction and ReinfarctionThe consensus document continues along the same lines in the definition of infarction “recurrence”; an infarction is considered recurrence when it occurs after the first 28 days after the first event.7 On the other hand, MI occurring in the first 28 days is considered a “reinfarction” when it is accompanied by new ECG changes and cTn elevation. If the levels are still elevated due to the first event, a greater than 20% increase is required for the new event to be considered a reinfarction. Analysis of creatine kinase MB isoform (CK-MB) is not useful or cost-effective in the differential diagnosis of myocardial reinfarction when the cTn can be determined.13
MYOCARDIAL INJURY AND INFARCTION ASSOCIATED WITH PROCEDURES, CHRONIC DISEASES, OR SPECIAL SITUATIONSThere are no updates in the section on myocardial injury associated with cardiac procedures (transcatheter aortic valve implantation or ablation). The diagnosis of MI is indicated in patients whose cTn elevation is accompanied by other criteria indicating ischemia, as occurs in type 5 MI.
The importance of the detection of noncardiac surgery-associated perioperative infarction is highlighted due to the prognostic impact. Evidence of ischemia is obligatory to catalog the MI event; when this is not present, the event is referred to as myocardial injury, which inherently confers worse prognosis.14 There is a lack of sufficient evidence to conclude if, for high-risk patients, determination of the preprocedural cTn value could contribute to the prognostic stratification. In addition, it is still difficult to determine the best time to proceed with the diagnosis and interventional treatment of a patient with perioperative infarction.
In the context of chronic kidney disease or heart failure, it is common to find small chronic elevations in cTn that are related to structural, inflammatory, or neurohormonal changes and that have mid-term prognostic impact. A novelty of the consensus document is to consider that, in these patients, when type 1 MI is suspected, the diagnosis should be established with the support of clinical signs and additional examinations (coronary angiography and imaging techniques).
TAKOTSUBO SYNDROMEThis new section in the document reflects the increased attention being paid to this entity. The syndrome should be suspected when the clinical manifestations and ECG abnormalities are disproportionate for the low cTn values and when left ventricular (LV) regional wall motion abnormalities do not correlate with a single coronary artery distribution. Although echocardiography may be useful, coronary angiography and ventriculography are usually necessary to confirm the diagnosis. Coronary artery disease may be present in up to 15% of patients but is not enough to explain the regional wall motion abnormalities. A QTc interval prolongation> 500ms during the acute phase and the recovery of LV function within 2 to 4 weeks can be useful in the differential diagnosis. Recovery of ventricular function is necessary to confirm the diagnosis of the syndrome. One aspect that the consensus document does not mention is if determination of N-terminal pro-brain natriuretic peptide (NT-proBNP) levels can be a useful prognostic marker to predict the risk of myocardial deterioration and recovery.14
MYOCARDIAL INFARCTION WITH NONOBSTRUCTIVE CORONARY ARTERIES (MINOCA)The growing scientific evidence on this entity has not been overlooked. It is important to note that MINOCA involves ischemia-related myocardial injury without stenosis ≥ 50% in a major epicardial vessel. In this setting, the causative mechanism should be investigated using intracoronary diagnostic techniques (optical coherence tomography [OCT]) because patients with MINOCA may appear to have type 1 MI and an incorrect diagnosis could lead to inadequate treatment. One aspect of the new definition worth highlighting is the recognition of MINOCA as an entity with its “own” criteria because, in the previous definition of MI, the diagnosis of MI could be considered in patients with myocardial injury without myocardial ischemia (sepsis, heart failure, chronic kidney disease).15
BIOCHEMICAL APPROACH FOR DIAGNOSING MYOCARDIAL INJURY AND INFARCTIONMI diagnosis requires the detection of variations in cTn levels, although their detection depends on the analytical method used, the blood flow, and the time of sample collection. The new consensus document stresses the advantages of hs-cTn over cTn. hs-cTn assays are characterized by their accurate measurement of cTn concentrations corresponding to the 99th percentile URL of the reference population and their ability to detect cTn concentrations lower than the 99th percentile in more than 50% of healthy individuals. Therefore, these assays allow the detection of lower cTn concentrations and smaller variations. Accordingly, the number of events diagnosed depends on the test used.
The document notes the lack of consensus regarding the definition of the 99th percentile of the reference population. The 99th percentile should be determined for each cTn analysis method according to the recommendations of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). hs-cTn tests must have a coefficient of variation <10% of the 99th percentile and hs-cTn assays with a coefficient of variation> 20% of this percentile should not be used. The indication to use different cutoff values according to age and sex in all hs-cTn tests is also controversial, even though both characteristics influence the URL determination. Because women typically have lower cTn values, they may be underdiagnosed.
Analysis of the hs-cTn has increased the diagnosis of MI by up to 30%, but clinical evaluation remains fundamental for the correct diagnosis of patients with elevated cTn due to another cause and to identify patients with high risk of MI but with variations in cTn values lower than those indicated. Variations may be more difficult to detect in patients who present early or late or in those whose values are moving from the ascending phase to the descending phase of the time-concentration curve for troponin. Analysis of the hs-cTn can shorten the time to diagnosis. Thus, strategies based on the detection of low and stable concentrations of hs-cTn are interesting because they have a high negative predictive value to rule out myocardial injury in less than 2hours in low-risk patients with normal ECG and symptoms of at least 2hours in duration.
For the diagnosis of MI, the cTn analysis should be repeated 3 to 6hours after the first and at least 1 value must be higher than the 99th percentile. However, samples may be required after 6hours in high-risk patients or those who present late after symptom onset (and are thus in the downslope of the curve) to detect significant changes that permit MI diagnosis.
Finally, the consensus document does not propose any algorithm of action in the emergency department for the detection of myocardial injury and MI, which would be a highly useful tool for health care professionals working in this field.
ELECTROCARDIOGRAPHIC DIAGNOSIS OF MYOCARDIAL INFARCTIONECG is essential for the diagnosis of MI and for the initial decision-making. There are few changes in this section from the previous document. When MI is suspected, an ECG should be recorded and interpreted in the first 10minutes after the first medical contact, preferably in the prehospital environment, and serially repeated in doubtful cases. Some poor prognostic indicators are highlighted, such as ST-segment deviation in multiple leads, ST-segment depression associated with elevation in aVR or V1, and the presence of abnormal Q waves, as well as the ability of conditions other than MI to show ST-segment deviation.
A prolonged new convex ST-segment elevation, particularly when associated with reciprocal ST-segment depression, usually indicates acute coronary occlusion. Although accurate, the inclusion of the term “convex”, not present in the previous consensus document, might be confusing because concave ST-segment elevation is not uncommon in ST-segment elevation MI.16
The same cutoff values used in the previous consensus document are again recommended to identify abnormal ST-segment elevation. Importantly, these values were obtained in a Caucasian population living in a specific area and cannot necessarily be extrapolated to the global population.16 Quite rightly, the consensus document emphasizes that these cutoff points should always be interpreted with consideration of the clinical context. In the presence of symptoms indicating ischemia, nonsignificant ST-segment elevation is often associated with acute coronary occlusion.17 A new and pertinent inclusion is the mention of an upsloping ST-segment depression pattern associated with prominent T waves in precordial leads and often with ST-segment elevation in aVR, produced by acute occlusion of the anterior descending coronary artery (the de Winter pattern),18 which should be considered an indication for immediate coronary angiography.
As in the previous consensus document, supplementary leads that help to detect MI caused by occlusion of the circumflex artery or right ventricle should be used when the 12-lead ECG is nondiagnostic. Likewise, specific values are recommended for the identification of pathological Q waves, which facilitates the homogenous interpretation of this finding. It is mentioned that new right bundle branch block in the absence of ST-segment elevation is frequently associated with poor flow in the culprit artery in patients with MI. In addition, for patients with pacemakers and suspected MI who are not pacemaker-dependent, temporary deactivation of the pacemaker is recommended to detect myocardial ischemia.
For the first time, the consensus document considers the phenomenon of electrical remodeling (electrical memory) in patients with tachyarrhythmia, pacemakers, or conduction disturbances that are accompanied by repolarization changes. Likewise, additional examinations are recommended to distinguish type 1 MI and type 2 MI in patients with recent-onset atrial fibrillation who present with cTn elevation and new ST-segment depression.
IMAGING TECHNIQUESThe role of noninvasive imaging is centered on the diagnosis and characterization of myocardial injury in MI.1 The imaging parameters considered in the MI evaluation are myocardial perfusion and viability, the thickness of the myocardium and its capacity for thickening and motion, and the effects of myocyte loss on the kinetics of paramagnetic or radiopaque contrast agents indicating myocardial fibrosis or scarring.
The high availability and versatility of transthoracic echocardiography make this imaging modality the technique of choice for the evaluation of patients with suspected MI: regional wall motion and thickness abnormalities can be detected when the ischemic area affects more than 20% of the myocardial thickness. Echocardiographic contrast agents permit the more accurate diagnosis of regional wall motion abnormalities, and tissue Doppler and strain imaging allow quantification of global and regional myocardial function. In addition, echocardiography can detect mechanical complications of MI in patients with hemodynamic deterioration and rule out other ominous prognostic diagnoses that can be confused with MI, such as aortic dissection or massive pulmonary embolism.
The value of other imaging techniques such as nuclear medicine, CMR, and CCTA is also summarized in this fourth definition of MI. The following aspects are novel: the evidence of the value of CCTA in the diagnosis of coronary artery disease in patients with low-to-intermediate risk and normal cTn at the time of admission to the chest pain unit, and the use of CMR to define the etiology of the myocardial injury. For the first time, a randomized study has compared the usefulness of CCTA with that of the standard diagnostic strategy that includes hs-cTn.19 CCTA did not reduce the length of hospital stay but was associated with a reduction in the use of additional diagnostic techniques and in the resulting costs. The high spatial resolution of CMR improves the characterization of MI because it detects myocardial edema, the myocardial area at risk, microvascular obstruction, intramyocardial hemorrhage, and infarct size, all parameters with important prognostic implications. In addition, in patients with possible MI but without coronary artery obstruction, CMR plays an important role in the diagnosis of other conditions, such as myocarditis, MI with spontaneous recanalization, and takotsubo syndrome.14
CMR is the modality of choice for late-presentation MI due to its ability to detect subendocardial scarring and differentiate ischemic cardiomyopathy from nonischemic cardiomyopathy, based on the late enhancement pattern when gadolinium is used as contrast agent.20 Echocardiography and nuclear medicine techniques provide important prognostic information for the management of patients with MI.
REGULATORY PERSPECTIVE ON MYOCARDIAL INFARCTION IN CLINICAL TRIALSThe document concludes with a brief analysis of the implications of 4UDMI for research. The authors highlight the benefit that the standardization of the definition will have for the comparison of clinical trial results, the pooling of equivalent events in safety analyses, and the reporting of events by clinical events committees. The importance of the lack of the comparability of diagnoses based on different types of cTn is emphasized. Accordingly, the authors recommend the use of a single type of cTn in central laboratories or, more realistically, to communicate in each center the multiple of the elevation above the 99th percentile indicated by each manufacturer as the threshold for MI diagnosis for each type of cTn used. In epidemiological studies, the appearance of Q waves on the ECG should be used to analyze silent/unrecognized MI, although there is no consensus on the optimal interval between ECGs, and annual tracings are considered prudent for populations with high rates of atherosclerotic events.
The document recognizes the possible psychological, labor, and health implications of the new definition for patients and their families, as well as for society regarding diagnosis-related coding, sick leave, proof of disability, care costs, population statistics, and other aspects. Thus, it is recommended that health care professionals be adequately informed of the new diagnostic criteria through the creation of educational materials and the appropriate adaptation of clinical practice guidelines. In general, 4UDMI will help in the performance of epidemiological studies and the international classification of diseases. However, it is recognized that the significant increase in the number of diagnosable MIs will require developed countries to make adjustments to the analysis of temporal trends, whereas more flexible diagnostic criteria should be used in countries with fewer resources, given their more limited access to biomarker tests.
Finally, an ambitious proposal, aimed at health care systems, is to move beyond the current diagnostic coding models and automatically incorporate the consensus document positions into the electronic medical record, using algorithms that include the analysis of biomarker results in a consistent and reliable manner, considering the specific type of cTn used in each environment, the value of the 99th percentile serving as the diagnostic threshold, and the sequence of values determined. A true challenge!
SEE RELATED ARTICLE: https://doi.org/10.1016/j.rec.2018.11.011
Rev Esp Cardiol. 2019;72(1):10-15
A. Sambola, et al. / Rev Esp Cardiol. 2019;72(1):10-15 11
12. A. Sambola, et al. / Rev Esp Cardiol. 2019;72(1):10-15
1885-5857/$ - see front matter © 2018 Sociedad Española de Cardiología. Published by Elsevier España, SL. All rights reserved.
CONFLICTS OF INTERESTNone declared.
SEC Working Group for the 2018 ESC Fourth Universal Definition of Myocardial Infarction: Antonia Sambola (Co-ordinator), Ana Viana-Tejedor (Co-ordinator), Héctor Bueno, José Antonio Barrabés, Victoria Delgado, Pilar Jiménez, Pablo Jorge Pérez, Francisco Javier Noriega, and Montserrat Vila.
Expert Reviewers for the 2018 ESC Fourth Universal Definition of Myocardial Infarction: Jaime Aboal, Alberto Bouzas, Salvatore Brugaletta, Albert Durán, José Juan Gómez de Diego, Felipe Hernández, Teresa López, Iñigo Lozano, Iván Núñez, Soledad Ojeda, Sandra Rosillo, and Juan Sanchis.
SEC Guidelines Committee: Fernando Alfonso, Borja Ibáñez, Fernando Arribas, Gemma Berga Congost, Héctor Bueno, Arturo Evangelista, Ignacio Ferreira-González, Manuel Jiménez Navarro, Francisco Marín, Leopoldo Pérez de Isla, Antonia Sambola, Rafael Vázquez, and Ana Viana-Tejedor.