We sought to determine the association of reciprocal change in the ST-segment with myocardial injury assessed by cardiac magnetic resonance (CMR) in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI).
MethodsWe performed CMR imaging in 244 patients who underwent primary PCI for their first STEMI; CMR was performed a median 3 days after primary PCI. The first electrocardiogram was analyzed, and patients were stratified according to the presence of reciprocal change. The primary outcome was infarct size measured by CMR. Secondary outcomes were area at risk and myocardial salvage index.
ResultsPatients with reciprocal change (n=133, 54.5%) had a lower incidence of anterior infarction (27.8% vs 71.2%, P < .001) and shorter symptom onset to balloon time (221.5±169.8 vs 289.7±337.3min, P=.042). Using a multiple linear regression model, we found that patients with reciprocal change had a larger area at risk (P=.002) and a greater myocardial salvage index (P=.04) than patients without reciprocal change. Consequently, myocardial infarct size was not significantly different between the 2 groups (P=.14). The rate of major adverse cardiovascular events, including all-cause death, myocardial infarction, and repeat coronary revascularization, was similar between the 2 groups after 2 years of follow-up (P=.92).
ConclusionsReciprocal ST-segment change was associated with larger extent of ischemic myocardium at risk and more myocardial salvage but not with final infarct size or adverse clinical outcomes in STEMI patients undergoing primary PCI.
Keywords
Acute transmural myocardial infarction induces ST-segment elevation, enlargement of the R wave, and widening of the QRS complex in electrocardiogram (ECG) leads directly related to the ischemic region.1,2 Moreover, leads not related to the ischemic area can show concurrent reciprocal ST-segment depression.3,4 Numerous studies have aimed to determine the clinical implications of reciprocal change on ECG. However, the clinical significance of reciprocal change on ECG such as ST-segment depression remote from the infarct site remains controversial, as reflected in a more extensive infarct size or the benign mirror phenomenon.4–8 Cardiac magnetic resonance (CMR) imaging can accurately describe myocardial scar change and the area at risk (AAR) in acute myocardial infarction. This technique can also be used to estimate the myocardial salvage index (MSI), as a meaningful CMR outcome parameter of acute reperfused myocardial infarction.9,10 Therefore, our study aimed to investigate whether reciprocal change in ST-segments is related to markers of myocardial injury, as assessed by CMR imaging and clinical outcomes in patients undergoing primary percutaneous coronary intervention (PCI) for ST-segment elevation myocardial infarction (STEMI).
METHODSStudy Population, Clinical Data, and Follow-upST-segment elevation myocardial infarction patients who visited our institution, a tertiary referral center from January 2008 to September 2013, were eligible if they had: a) ST-segment elevation in 2 or more contiguous leads on ECG as standard definition; and b) had undergone successful primary PCI and CMR imaging. Patients with the following were excluded: admission for more than 12hours after symptom onset, previous myocardial infarction, or previous coronary revascularization (coronary artery bypass graft or PCI). Baseline clinical data from the dedicated registry, including past medical history, presence of risk factors, medications, angiographic and procedural data, and clinical outcomes, were recorded prospectively by research coordinators. This study was approved by the local institutional review board and waived the requirement for informed consent.
Percutaneous coronary intervention was performed using standard intervention techniques.11 The decision to pursue invasive treatment, as well as determining the access site, stent type for intravascular ultrasound, and use of glycoprotein IIb/IIIa receptor inhibitors, was left to the physician's discretion. Myocardial blush grade was evaluated using a final angiogram, as described previously.12 All interventions and procedural anticoagulations were performed in accordance with current standard guidelines.13
The primary outcome was myocardial infarct size (% of the left ventricle [LV] mass) measured by CMR. Secondary outcomes were AAR (% of LV mass), MSI (%), microvascular obstruction, and hemorrhagic infarction. Major adverse cardiovascular events were defined as a composite of all-cause death, myocardial infarction, and repeat coronary revascularization.
Definition of Electrocardiogram AnalysisA STEMI diagnosis was made when ECG change fit the standard definition of new ST elevation at the J-point in at least 2 contiguous leads of at least ≥ 2mm (0.2mV) in men or ≥ 1.5mm (0.15mV) in women in leads V2-V3 and/or of ≥ 1mm (0.1mV) in other contiguous chest leads or the limb leads plus one of the following 2 criteria: duration of chest pain more than 30minutes or elevation of serum cardiac enzyme markers.14 A reciprocal change was defined as the presence of a 0.1-mV depression 80 msec after the J-point in 2 or more adjacent leads far from the leads exhibiting ST-segment elevation.15 Patients were stratified according to the presence or absence of reciprocal ECG changes.
Cardiac Magnetic Resonance Imaging Acquisition and AnalysisCardiac magnetic resonance was performed using a 1.5-T scanner (Achieva; Philips Medical Systems, Best, The Netherlands) with a SENSE cardiac coil according to our laboratory protocol.16 Images were acquired using electrocardiographic gating and expiratory breath holds. The CMR protocol consisted of cine, T2-weighted images, first-pass perfusion, and late-gadolinium enhancement imaging. Cine imaging was carried out based on balanced steady-state free precession sequences along the long and short axes from the apex to the base of the LV. Next, T2Ws were acquired in the cardiac short-axis direction using a dark-blood T2W inversion-recovery fast-spin echo sequence. First-pass perfusion imaging was obtained with the T1-weighted dynamic sequence (turbo field echo with SENSE, repetition time/echo time=2.6/1.3ms) after intravenous infusion of gadolinium-diethylenetriamine penta-acetic acid (Gd-DTPA, Magnevist; Bayer Schering Pharma, Berlin, Germany; 0.15 mmol/kg weight in total amount at 3mL/sec). Slice thickness was set to 6mm with a field of view of 40cm × 40cm and an image matrix of 128 × 128. Images of 4 locations for every 2 heartbeats were acquired for 40 phases.
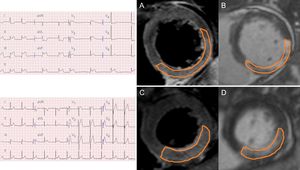
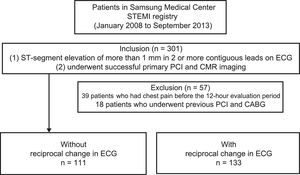
The CMR images were analyzed using validated software (ARGUS; Siemens Medical System, Erlangen, Germany) at our MRI core laboratory by 2 experienced radiologists who were blinded to the clinical information. After acquisision of the short-axis images at the end of diastole and at the end of systole, endocardial borders were traced manually. Left ventricle, end-diastolic volume, end-systolic volume, and ejection fraction were calculated using the Simpson rule. The infarct volume was quantified as the sum of the area with late-gadolinium enhancement within each segment of the short-axis images multiplied by slice thickness to cover the entire LV.17 The infarct area was traced by the visual border detection via a manual drawing method using commercialized analysis software. Microvascular obstruction was defined and planimetered manually as a hypointense core within an infarcted myocardium on late-gadolinium enhancement images. The AAR mass was defined from T2W images as the myocardial mass with signal intensity ≥ 2 standard deviations above remote. The AAR mass was then normalized to the LV myocardial mass to estimate the AAR percentage. Intramyocardial hemorrhage was identified and planimetered manually as a hypointense core within the AAR on T2W images. Myocardial salvage was calculated as (AAR mass-infarct mass), and MSI was calculated as (myocardial salvage/AAR mass) and was expressed as a percentage.9,18Figure 1 shows a representative CMR image of reperfused nonanterior STEMI with or without reciprocal ST change.
Representative cardiac magnetic resonance images from a study of nonanterior ST-segment elevation myocardial infarction with and without reciprocal change. A: a short-axis slice of a T2-weighted image with reciprocal change. B: a short-axis slice of a late-gadolinium enhancement image with reciprocal change. C: a short-axis slice of a T2-weighted image without reciprocal change. D: a short-axis slice of a late-gadolinium enhancement image without reciprocal change.
Continuous variables were compared using Student t test or the Wilcoxon rank-sum test where applicable and are presented as mean±standard deviation or median with interquartile range [IQR]. Categorical data were tested using the Fisher exact test or the chi-square test, as appropriate. Covariates that appeared to be relevant by virtue of having P-values < .2 on univariate analysis or were otherwise clinically relevant were included in our risk factor adjustment model, using the multiple linear regression model. To balance the underlying demographic and clinical characteristics, we also considered the propensity score method. Propensity scores were estimated using a multiple logistic regression model that included all variables presented in Table 1. The covariate balance was assessed by comparing the absolute standardized differences in covariates between the 2 groups (Table of the supplementary material). In the propensity score-matched patients, continuous variables as primary outcome and secondary outcomes of CMR were compared with a paired t test or the Wilcoxon signed-rank test. Categorical variables were compared with the McNemar or Bowker test of symmetry, as appropriate. We checked the residual plots thoroughly, including Cook's distance and the normal probability plots, and confirmed them to be satisfactory before adopting any final model results.
Baseline Clinical and Angiographic Characteristics
| Variables | With reciprocal change | Without reciprocal change | P |
|---|---|---|---|
| n=133 | n=111 | ||
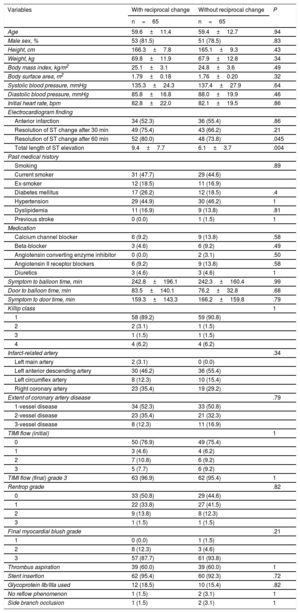
| Age | 59.5±11.9 | 58.4±11.6 | .5 |
| Male sex, % | 101 (75.9) | 92 (82.9) | .21 |
| Height, cm | 165.4±8.1 | 166.1±7.9 | .47 |
| Weight, kg | 68.8±13.3 | 67.6±11.3 | .49 |
| Body mass index, kg/m2 | 25.0±3.6 | 24.4±3.2 | .19 |
| Body surface area, m2 | 1.77±0.20 | 1.76±0.18 | .71 |
| Systolic blood pressure, mmHg | 129.7±26.8 | 139.4±27.5 | .09 |
| Diastolic blood pressure, mmHg | 81.9±18.0 | 89.7±19.1 | .006 |
| Initial heart rate, bpm | 77.0±22.4 | 81.4±18.3 | .001 |
| Electrocardiogram finding | |||
| Anterior infarction | 37 (27.8) | 79 (71.2) | < .001 |
| Resolution of ST change after 30 min | 94 (70.7) | 76 (68.5) | .78 |
| Resolution of ST change after 60 min | 102 (76.7) | 83 (74.8) | .77 |
| Total length of ST elevation | 8.1±6.3 | 7.4±5.1 | .31 |
| Past medical history | |||
| Smoking | .58 | ||
| Current smoker | 64 (48.1) | 55 (49.5) | |
| Ex-smoker | 20 (15.0) | 21 (18.9) | |
| Diabetes mellitus | 37 (27.8) | 18 (16.2) | .032 |
| Hypertension | 68 (51.1) | 43 (38.7) | .07 |
| Dyslipidemia | 26 (19.55) | 14 (12.60) | .17 |
| Previous stroke | 5 (3.8) | 2 (1.8) | .46 |
| Medication | |||
| Calcium channel blocker | 19 (14.3) | 11 (9.9) | .33 |
| Beta-blocker | 7 (5.3) | 8 (7.2) | .6 |
| Angiotensin converting enzyme inhibitor | 0 (0.0) | 2 (1.8) | .21 |
| Angiotensin II receptor blockers | 13 (9.8) | 11 (9.9) | 1 |
| Diuretics | 6 (4.5) | 3 (2.7) | .52 |
| Symptom to balloon time, min | 221.5±169.8 | 289.7±337.3 | .042 |
| Symptom to door time, min | 147.9±139.3 | 179.7±176.0 | .12 |
| Troponin I | 114.68±113.93 | 90.61±117.79 | .11 |
| Killip class | .34 | ||
| 1 | 116 (87.2) | 102 (91.9) | |
| 2 | 2 (1.5) | 3 (2.7) | |
| 3 | 5 (3.8) | 1 (0.9) | |
| 4 | 10 (7.5) | 5 (4.5) | |
| Infarct-related artery | < .001 | ||
| Left main artery | 2 (1.5) | 0 (0.0) | |
| Left anterior descending artery | 36 (27.1) | 78 (70.3) | |
| Left circumflex artery | 20 (15.0) | 12 (14.6) | |
| Right coronary artery | 75 (56.4) | 21 (18.9) | |
| Proximal occlusion of infarct-related artery | 84 (63.2) | 66 (59.5) | .60 |
| Extent of coronary artery disease | .51 | ||
| 1-vessel disease | 68 (51.1) | 65 (58.6) | |
| 2-vessel disease | 45 (33.8) | 33 (29.7) | |
| 3-vessel disease | 20 (15.0) | 13 (11.7) | |
| TIMI flow (initial) | .06 | ||
| 0 | 107 (80.5) | 74 (66.7) | |
| 1 | 9 (6.8) | 8 (7.2) | |
| 2 | 9 (6.8) | 13(11.7) | |
| 3 | 8 (6.0) | 16 (14.4) | |
| TIMI flow (final) | 1 | ||
| 2 | 6 (4.5) | 5 (4.5) | |
| 3 | 127 (95.5) | 106 (95.5) | |
| Rentrop grade | .99 | ||
| 0 | 61 (45.9) | 50 (45.0) | |
| 1 | 51 (38.3) | 44 (39.6) | |
| 2 | 20 (15.0) | 16 (14.4) | |
| 3 | 1 (0.8) | 1 (0.9) | |
| Final myocardial blush grade | .38 | ||
| 1 | 0 (0.0) | 1 (0.9) | |
| 2 | 11 (8.3) | 6 (5.4) | |
| 3 | 122 (91.7) | 104 (93.7) | |
| Thrombus aspiration | 95 (74.1) | 60 (54.1) | .007 |
| Stent insertion | 128 (96.2) | 106 (95.5) | 1 |
| Glycoprotein IIb/IIIa used | 28 (21.15) | 16 (14.45) | .19 |
| No reflow phenomenon | 13 (9.8) | 2 (1.8) | .014 |
| Side branch occlusion | 2 (1.50) | 4 (3.65) | .42 |
TIMI, Thrombolysis In Myocardial Infarction.
Values are presented as mean±standard deviation or as No. (%).
All tests were 2-tailed, and P < .05 was considered statistically significant. All analyses were performed using the Statistical Analysis Software package (SAS version 9.2, SAS Institute, Cary, North Carolina, United States).
RESULTSPatient Baseline CharacteristicsOf the 301 registered patients, 39 who had chest pain before the 12-hour evaluation period and 18 who underwent previous PCI and coronary artery bypass grafting were excluded from our analysis. A total of 244 patients met our inclusion criteria for undergoing successful primary PCI and CMR imaging. These patients were divided into 2 groups: those with reciprocal change (n=133) and those without reciprocal change (n=111; Figure 2).
The baseline clinical and angiographic characteristics by patient group are summarized in Table 1. Patients with reciprocal change had a higher frequency of diabetes mellitus (27.8% vs 16.2%; P=.032) and nonanterior infarction (72.2% vs 28.8%; P < .001) than those without. Pain onset to balloon time was shorter in patients with reciprocal change (221.5±169.8 vs 289.7±337.3 min; P=.042). Patients with reciprocal change had a higher incidence of baseline Thrombolysis In Myocardial Infarction flow of grade 0, thrombus aspiration, and no reflow during PCI compared with those without reciprocal change. Coronary angiography a found similar distribution of contralateral disease between the 2 groups (38.3% vs 35.1%; P=.69). The baseline clinical and angiographic characteristics in propensity score-matched patients are shown in Table 2.
Baseline Clinical and Angiographic Characteristics in Propensity Score-matched Patients
| Variables | With reciprocal change | Without reciprocal change | P |
|---|---|---|---|
| n=65 | n=65 | ||
| Age | 59.6±11.4 | 59.4±12.7 | .94 |
| Male sex, % | 53 (81.5) | 51 (78.5) | .83 |
| Height, cm | 166.3±7.8 | 165.1±9.3 | .43 |
| Weight, kg | 69.8±11.9 | 67.9±12.8 | .34 |
| Body mass index, kg/m2 | 25.1±3.1 | 24.8±3.6 | .49 |
| Body surface area, m2 | 1.79±0.18 | 1.76±0.20 | .32 |
| Systolic blood pressure, mmHg | 135.3±24.3 | 137.4±27.9 | .64 |
| Diastolic blood pressure, mmHg | 85.8±16.8 | 88.0±19.9 | .46 |
| Initial heart rate, bpm | 82.8±22.0 | 82.1±19.5 | .86 |
| Electrocardiogram finding | |||
| Anterior infarction | 34 (52.3) | 36 (55.4) | .86 |
| Resolution of ST change after 30 min | 49 (75.4) | 43 (66.2) | .21 |
| Resolution of ST change after 60 min | 52 (80.0) | 48 (73.8) | .045 |
| Total length of ST elevation | 9.4±7.7 | 6.1±3.7 | .004 |
| Past medical history | |||
| Smoking | .89 | ||
| Current smoker | 31 (47.7) | 29 (44.6) | |
| Ex-smoker | 12 (18.5) | 11 (16.9) | |
| Diabetes mellitus | 17 (26.2) | 12 (18.5) | .4 |
| Hypertension | 29 (44.9) | 30 (46.2) | 1 |
| Dyslipidemia | 11 (16.9) | 9 (13.8) | .81 |
| Previous stroke | 0 (0.0) | 1 (1.5) | 1 |
| Medication | |||
| Calcium channel blocker | 6 (9.2) | 9 (13.8) | .58 |
| Beta-blocker | 3 (4.6) | 6 (9.2) | .49 |
| Angiotensin converting enzyme inhibitor | 0 (0.0) | 2 (3.1) | .50 |
| Angiotensin II receptor blockers | 6 (9.2) | 9 (13.8) | .58 |
| Diuretics | 3 (4.6) | 3 (4.6) | 1 |
| Symptom to balloon time, min | 242.8±196.1 | 242.3±160.4 | .99 |
| Door to balloon time, min | 83.5±140.1 | 76.2±32.8 | .68 |
| Symptom to door time, min | 159.3±143.3 | 166.2±159.8 | .79 |
| Killip class | 1 | ||
| 1 | 58 (89.2) | 59 (90.8) | |
| 2 | 2 (3.1) | 1 (1.5) | |
| 3 | 1 (1.5) | 1 (1.5) | |
| 4 | 4 (6.2) | 4 (6.2) | |
| Infarct-related artery | .34 | ||
| Left main artery | 2 (3.1) | 0 (0.0) | |
| Left anterior descending artery | 30 (46.2) | 36 (55.4) | |
| Left circumflex artery | 8 (12.3) | 10 (15.4) | |
| Right coronary artery | 23 (35.4) | 19 (29.2) | |
| Extent of coronary artery disease | .79 | ||
| 1-vessel disease | 34 (52.3) | 33 (50.8) | |
| 2-vessel disease | 23 (35.4) | 21 (32.3) | |
| 3-vessel disease | 8 (12.3) | 11 (16.9) | |
| TIMI flow (initial) | 1 | ||
| 0 | 50 (76.9) | 49 (75.4) | |
| 1 | 3 (4.6) | 4 (6.2) | |
| 2 | 7 (10.8) | 6 (9.2) | |
| 3 | 5 (7.7) | 6 (9.2) | |
| TIMI flow (final) grade 3 | 63 (96.9) | 62 (95.4) | 1 |
| Rentrop grade | .82 | ||
| 0 | 33 (50.8) | 29 (44.6) | |
| 1 | 22 (33.8) | 27 (41.5) | |
| 2 | 9 (13.8) | 8 (12.3) | |
| 3 | 1 (1.5) | 1 (1.5) | |
| Final myocardial blush grade | .21 | ||
| 1 | 0 (0.0) | 1 (1.5) | |
| 2 | 8 (12.3) | 3 (4.6) | |
| 3 | 57 (87.7) | 61 (93.8) | |
| Thrombus aspiration | 39 (60.0) | 39 (60.0) | 1 |
| Stent insertion | 62 (95.4) | 60 (92.3) | .72 |
| Glycoprotein IIb/IIIa used | 12 (18.5) | 10 (15.4) | .82 |
| No reflow phenomenon | 1 (1.5) | 2 (3.1) | 1 |
| Side branch occlusion | 1 (1.5) | 2 (3.1) | 1 |
TIMI, Thrombolysis In Myocardial Infarction.
Values are presented as mean±standard deviation or as No. (%).
Patients underwent CMR at a median of 3 days [IQR 3-5] after primary PCI, and there was no a significant difference between 2 groups (4.9±5.7 vs 5.0±5.5 days; P=.93). Infarct size, the primary outcome, was not significantly different between patients without and with reciprocal change (median [IQR]: 18.33% [12.34–27.65] vs 17.95% [11.44–25.49]; P=.35). Although patients with reciprocal changes had significantly higher AAR than those without (37.24% [27.83–46.82] vs 33.28% [20.07–44.27]; P=.05), MSI was not significantly different between the groups (48.57% [34.65–58.69] vs 44.63% [33.15–56.88]; P=.27). The other CMR parameters, including LV ejection fraction (P=.46), LV end-diastolic volume (P=.89), LV end-systolic volume (P=.83), LV stroke volume (P=.46), LV cardiac output (P=.56), LV mass (P=.66), the presence of microvascular obstruction (P=.15), and hemorrhagic infarction (P=.12), were not significantly different between the groups (Table 3). In a propensity score-matched subset data, CMR outcome parameters were not significantly different (Table 4).
Cardiac Magnetic Resonance Imaging Parameters
| Variables | With reciprocal change | Without reciprocal change | P |
|---|---|---|---|
| n=133 | n=111 | ||
| LVEDV, mL | 142.70 [121.71-164.48] | 143.04 [123.02-163.04] | .89 |
| LVESV, mL | 66.55 [50.04-82.18] | 67.96 [49.43-82.62] | .83 |
| LV ejection fraction, % | 53.80 [45.05-60.61] | 53.81 [46.93-60.92] | .46 |
| LV stroke volume, mL | 72.67 [63.28-85.49] | 75.62 [64.2-85.79] | .87 |
| LV cardiac output, L/min | 4.93 [4.26-5.61] | 5.13 [4.55-5.88] | .56 |
| LV mass, g | 100.70 [84.33-118.61] | 100.45 [78.81-116.45] | .66 |
| Infarct size/LV, % of LV mass | 18.33 [12.34-27.65] | 17.95 [11.44-25.49] | .35 |
| Area at risk/LV, % of LV mass | 37.24 [27.83-46.82] | 33.28 [20.07-44.27] | .05 |
| Myocardial salvage index, % | 48.57 [34.65-58.69] | 44.63 [33.15-56.88] | .27 |
| Presence of microvascular obstruction | 84 (63.2) | 60 (54.1) | .15 |
| Presence of hemorrhagic infarction | 65 (48.9) | 43 (38.7) | .12 |
| Variables | Slope estimate* | Standard error* | P* |
|---|---|---|---|
| Infarct size/LV, % of LV mass | 2.11 | 1.43 | .14 |
| Area at risk/LV, % of LV mass | 6.97 | 2.20 | .002 |
| Myocardial salvage index, % | 5.47 | 2.66 | .041 |
LV, left ventricle; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume.
Unless otherwise indicated, values are presented as median [interquartile range] or No. (%).
Results were from the multiple linear regression model, including propensity score and the covariates such as reciprocal change, sex, body mass index, systolic blood pressure, diastolic blood pressure, symptom to balloon time (min), previous stroke, Killip class, infarct-related artery, Rentrop grade, and thrombus aspiration for confounding adjustment.
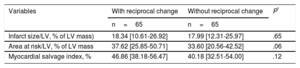
Parameters of Cardiac Magnetic Resonance Imaging as Outcomes in Propensity Score-matched Subset Data
| Variables | With reciprocal change | Without reciprocal change | P* |
|---|---|---|---|
| n=65 | n=65 | ||
| Infarct size/LV, % of LV mass) | 18.34 [10.61-26.92] | 17.99 [12.31-25.97] | .65 |
| Area at risk/LV, % of LV mass | 37.62 [25.85-50.71] | 33.60 [20.56-42.52] | .06 |
| Myocardial salvage index, % | 46.86 [38.18-56.47] | 40.18 [32.51-54.00] | .12 |
LV, left ventricle.
Values are presented as median with interquartile range [Q1-Q3].
After the performance of multiple linear regressions that included propensity scores and other adjustments for confounding variables, patients with reciprocal change had a larger extent of AAR (slope estimate=6.97, standard error [SE]=2.20, P=.002), and a greater MSI (slope estimate=5.47, SE=2.64, P=.04) compared with those without. Consequently, myocardial infarct size was not significantly different between the 2 groups (slope estimate=2.11, SE=1.43, P=.14).
The overall reciprocal change in ST depression was associated with infarct size (slope estimate=0.57, SE=0.25, P=.021) and AAR (slope estimate=1.02, SE=0.35, P=.004), according to our analysis.
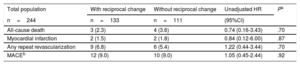
Clinical OutcomesThe cumulative clinical outcomes of the study patients are summarized in Table 5. The median length of follow-up was 26.7 [IQR: 12.8–40.9] months and was not significantly different between patients without reciprocal change (28.2 [IQR: 11.1–42.8] months) and those with reciprocal change (25.9 [IQR: 13.3–38.9] months, P=.54).
Clinical Outcomes of the Group With Reciprocal Change Compared With the Group Without Reciprocal Change During the Follow-up Period
| Total population | With reciprocal change | Without reciprocal change | Unadjusted HR | Pa |
|---|---|---|---|---|
| n=244 | n=133 | n=111 | (95%CI) | |
| All-cause death | 3 (2.3) | 4 (3.6) | 0.74 (0.16-3.43) | .70 |
| Myocardial infarction | 2 (1.5) | 2 (1.8) | 0.84 (0.12-6.00) | .87 |
| Any repeat revascularization | 9 (6.8) | 6 (5.4) | 1.22 (0.44-3.44) | .70 |
| MACEb | 12 (9.0) | 10 (9.0) | 1.05 (0.45-2.44) | .92 |
95%CI, 95% confidence interval; HR, hazard ratio; MACE, major adverse cardiovascular events.
Unless otherwise indicated, data are expressed as No. (%)
The log-rank test indicated no significant differences in event times to clinical outcomes between the 2 groups (Table 5). The major adverse cardiovascular events occurred in 9% of patients with or without reciprocal change and were not significantly different (HR, 1.05; 95%CI, 0.45–2.44; P=.92; Table 5).
DISCUSSIONWe compared CMR imaging findings and clinical outcomes between patients with and without reciprocal ECG change in the context of STEMI. The major findings of this study were as follows: a) patients with reciprocal change had a higher frequency of nonanterior infarction and shorter total ischemic time than those without; b) reciprocal ST-segment change was associated with a larger extent of at-risk ischemic myocardium, but also more salvaged myocardium. Final infarct size, as assessed by CMR imaging, was not associated with reciprocal ST-segment change, and c) there was no significant difference in major adverse cardiovascular event-free survival according to the presence or absence of reciprocal ECG change.
The underlying mechanism of concomitant ST-segment depression in patients with STEMI is highly controversial. Previous studies have found that reciprocal change in ST-segment depression was a main electrocardiographic finding across a broad spectrum of patients with regard to myocardial infarction size, severity of coronary artery disease, and the amount of jeopardized myocardium.19–21 Therefore, there is a need for better evaluations that allow for a more accurate gradation of risk. Other researchers have speculated that reciprocal ST-segment depression may reflect a more extensive infarction,5,22,23 additional ischemia beyond the infarct zone,6,23,24 more diffuse coronary artery disease,25,26 or simply a benign mirror projection of the ST-segment elevation without significant clinical relevance.8,27 Most of the previous studies cited above investigated the association of reciprocal change with myocardial injury through cardiac enzyme assessment, positron emission tomography, or ventriculography.7,28–30 However, the pathologic mechanisms underlying this association are still unclear. Cardiac magnetic resonance imaging can accurately estimate the extent of myocardial injury and salvaged myocardium and thus offer a better understanding of reciprocal ST-segment depression in STEMI patients. Therefore, we assessed the relationship between reciprocal ECG change and myocardial injury as determined by CMR in STEMI patients undergoing primary PCI.
In this study, reciprocal ST-segment change was associated with a larger risk extent of ischemic myocardium and more salvaged myocardium, as assessed by CMR imaging. The results of this study are consistent with those of a previous study, which reported that 35 STEMI patients with reciprocal changes on admission ECG had a larger AAR mass and higher extent of salvaged myocardium according to CMR than those without.31 In both studies, no relationship was found between reciprocal change and myocardial infarct size. However, previous studies failed to explain why patients with reciprocal change had larger AAR with similar infarct size compared with those without.
We evaluated the impact of reciprocal ECG change on clinical outcomes between the study groups and found no significant differences in terms of major adverse cardiovascular events. Considering that myocardial infarct size is the most important prognostic factor for STEMI patients and that reciprocal change could not predict left ventricular dysfunction or adverse clinical outcomes, it seems that reciprocal change is not a reliable ischemia marker.15,32 In previous studies,30,33,34 the reciprocal ECG change was associated with larger infarction size. Increased AAR, not infarct size, in those with reciprocal ECG changes suggested a larger exposed ischemic mass at the time of the ECG in the era of primary PCI. The extent of AAR on T2-weighted imaging, as the myocardium to be infarcted prior to opening of the infarct-related artery, could be viable in the absence of complete necrosis. Our study found that compared with patients without reciprocal ECG changes, those with reciprocal ECG changes had a greater ischemic myocardium at risk, and a shorter total ischemic time. In addition, reciprocal ECG change was associated with a larger salvaged myocardium, but similar infarct size following primary PCI. Therefore, reciprocal ECG changes could be a marker of a larger ischemic myocardium at risk with viability in the early stage of STEMI, and suggest that there is a possibility of improving myocardial salvage following prompt and timely reperfusion therapy.
Comparing outcomes across patients with reciprocal change, however, the degree of total summation of ST depression with reciprocal change was significantly associated with a larger AAR (slope estimate=1.02, SE=0.35, P=.004). Initial total summation of ST depression was also associated with a larger infarct size (slope estimate=0.57, SE=0.25, P=.021). Consequently, this did not have a decisive effect on interventions for myocardial salvage. Whether these subsets of patients with reciprocal change allow us to predict outcomes from additional therapeutic interventions needs to be determined in future prospective studies.
LimitationsThis study has several limitations. This was a nonrandomized observational study, and our results may have been significantly affected by confounding factors. Our study included data from a single center and had a limited number of patients, despite being the largest CMR study to date that assessed AAR values, infarct size, myocardial salvage, and MSI. Based on the inclusion and exclusion criteria of this single-center study and the treatment protocols used in specialized high-volume hospitals, our results may not be not generalizable to other STEMI populations, such as late STEMI presenters (> 12hours). Additionally, the predominantly male study populations limited the generalizability of the results. The patients were included over a relatively long time period during which therapeutic and guidelines changes could have occurred that could have influenced the results.
Additionally, the 2 groups were qualitatively quite different, and as a result, our participant loss was fairly significant when we attempted to match the groups. The number of pairs matched was extremely limited. Therefore, matched analysis was used to compare the results with those of the original unmatched cases. Had the number of unmatched cases been small, we would have attempted to identify the clinical reasons why they became unmatched.
Whether the real AAR is accurately delineated by T2-weighted CMR has been a matter of intense debate.35,36 Indeed, recent experimental data suggest that T2 may represent the actual infarct size rather than the real AAR,37 or may be influenced by the time between the acute event and image acquisition.38 It might be that hyperintense areas in T2-weighted sequences may not reflect the real AAR, and therefore provide biased estimates of myocardial salvage and inaccurate adjustments in statistical models. It has been recently suggested that edema might a dynamic phenomenon, and therefore unstable, during the first week after myocardial infarction. As opposed to early assessment alone, it might be crucial to perform CMR in a systematic narrow time window. For myocardial salvage measurement, we considered that early assessment of the AAR was crucial because myocardial edema was maximal and constant during the first week after infarction and decreased thereafter.39,40 Despite the lack of difference in the time interval from primary PCI to CMR between the groups (4.9±5.7 vs 5.0±5.5 days; P=.93), AAR size may vary significantly if measured 1 to 40 days after primary PCI, and the patients were not scanned at such a consistent narrow time window.
We could not completely rule out possible overestimation of the infarct size caused by the presence of edema on CMR within first 5 days. However, it would not have affected the results of our study because there was no significant difference in the time interval from primary PCI to CMR between the 2 groups.
The low event rate in our study population and the wide confidence intervals do not provide convincing evidence of poorer outcomes. In addition, the low incidence of events could be caused by a recruitment bias, with the inclusion of patients with nonsevere myocardial infarction in this CMR registry, as it showed a higher than expected incidence of Killip class I (87.2% and 91.9% in patients with and without reciprocal change). While adverse clinical events were not centrally adjudicated in our registries, all events were identified by the patients’ physicians and confirmed by the hospital's principal investigator.
CONCLUSIONSReciprocal ECG change was not associated with larger myocardial infarct size, but was associated with greater extent of ischemic myocardium and more myocardial salvage as assessed by CMR imaging in STEMI patients undergoing primary PCI. The presence of reciprocal ECG change might facilitate early diagnosis and reperfusion of STEMI, which in turn, would improve myocardial salvage.
CONFLICTS OF INTERESTNone declared.
- –
Numerous studies have aimed to determine the clinical implications of reciprocal change on ECG. However, the clinical significance of reciprocal change on ECG as ST-segment depression remote from the infarct site remains controversial, as reflected in a more extensive infarct size or the benign mirror phenomenon. Cardiac magnetic resonance imaging can accurately describe myocardial scar change and AAR in acute myocardial infarction. It can also be used to estimate the MSI, as a meaningful CMR outcome parameter of acute reperfused myocardial infarction.
- –
Patients with reciprocal change had a higher frequency of nonanterior infarction and shorter total ischemic time than those without.
- –
Reciprocal ST-segment change was associated with a larger extent of at-risk ischemic myocardium, but also more salvaged myocardium. Final infarct size, as assessed by CMR imaging, was not associated with reciprocal ST-segment change.
- –
There was no significant difference in major adverse cardiovascular event-free survival according to the presence or absence of reciprocal ECG change.
- –
The presence of reciprocal ECG change might facilitate early diagnosis and reperfusion of STEMI would improve myocardial salvage.