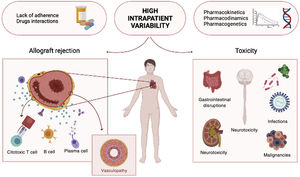
Heart transplant (HT) remains the gold standard treatment for advanced heart failure and other end-stage cardiac diseases.1 Although the success of HT largely depends on careful recipient and donor selection, long-term outcomes also depend on achieving an exquisite balance in immunosuppression. Over the last few years, while advances in patient management regarding surgical procedures and postoperative care have improved short-term survival, long-term survival has not shown an equivalent increase.2 From an allograft-centered point of view, the reasons for such static survival are diverse, yet episodes of clinical or even subclinical acute rejection and coronary allograft vasculopathy are the most recognized mechanisms that lead to chronic graft dysfunction. In addition, the secondary effects of immunosuppression and the toxicity of immunomodulating agents are critical for overall patient survival, irrespective of allograft viability (figure 1).
Current up-to-date treatment in solid organ transplant (SOT) focuses on avoiding direct and indirect T-lymphocyte responses by combining different immunomodulating agents with distinct mechanisms of action tackling T-cell blockade.3 In depth, when alloantigens are recognized by the T-cell membrane receptor, a cascade of reactions is set in motion involving several enzymes, especially calcineurin. This, along with other proteins, stimulates the formation of interleukin 2, the main autocrine activator of T cells.4 The introduction of calcineurin inhibitors (CNI) such as cyclosporine (CsA) and tacrolimus (TAC) has significantly improved both graft and recipient survival and these drugs are currently considered the cornerstone of immunosuppressive therapy in HT. The current approach to SOT is combining a CNI, generally TAC, with other adjuvant immunomodulators such as antiproliferative agents (mycophenolate mofetil), mTOR inhibitors (everolimus and sirolimus) or azathioprine and steroids, promoting additive effects by combining different mechanisms of action.
Immunosuppressive medications are considered “critical-dose drugs” because of their narrow therapeutic range, their concentration-effect relationship and high blood concentration variability and they therefore require close therapeutic drug monitoring. Underexposure may lead to risk of graft rejection whereas overexposure is associated with the risk of adverse effects such as nephrotoxicity, neurotoxicity, malignancies, and infections. The complexity is such that identical doses of CNI in different patients may result in different blood exposures and therefore in different effects and in the same person and an identical dose may result in different whole blood levels. These constant fluctuations in drug blood concentrations constitute a major challenge to ensure stable therapeutic steady-state plasma concentrations. For a precise therapeutic monitoring of immunomodulating agents, the 24-hour area under the curve (AUC)—which represents how the blood drug concentration changes over time—is considered the most representative pharmacokinetic marker of blood drug exposure and clinical effect. However, in most transplant centers, clinicians rely on trough concentration (CO) measurements and AUC is relegated to a more research-based setting as it is a more time consuming, resource-intensive and costly process. Despite being practical and accessible, such an approach is known to be flat and imprecise and to generate high fluctuations in blood concentrations in the same patient, with the same drug dose (intrapatient variability [IPV]). In fact, the association between TAC blood concentrations, elevated IPV and transplant outcomes have been demonstrated in several studies and the control of the sources of such variation are considered a strong opportunity to improve outcomes.5,6
The most important source of IPV is widely considered to be lack of treatment adherence. However, whole blood concentrations and effectiveness are also affected by multiple factors such as age, ethnicity, genetic polymorphisms, liver and kidney function, presystemic metabolism, concomitant medication interaction and herbs and food constituents that impact on drug pharmacokinetics, pharmacodynamics and pharmacogenetics.5.7,8 Although the exact mechanism by which intraindividual changes in immunosuppressant exposure influence graft integrity is still unknown, a high IPV in TAC exposure has been associated with poor graft outcomes, caused by episodes of acute and chronic rejection, the development of donor-specific anti-HLA (human leucocyte antigens) antibodies, and progressive fibrotic damage to the graft.8–11 Therefore, the routine evaluation of these fluctuations using IPV by analyzing whole blood concentrations periodically may be a useful biomarker to closely monitor HT patients.8
Accordingly, the work recently presented in Revista Española de Cardiología by González-Vílchez et al.12 contributes meaningfully to evaluate the impact of CNI IPV in HT recipients. Briefly, using the Spanish Heart Transplant Registry—a national multicenter database containing information of both donors and HT recipients since 1984—the authors performed an observational retrospective longitudinal study to examine the clinical impact of CNI variability on HT patient outcomes. To date, this is possibly the largest study of this type and the first to compare the impact of variability in each CNI. According to the registry, 3387 patients underwent a HT procedure between 2000 and 2014, of which 1581 were managed with either immediate release TAC (33.3%), long release TAC (16.7%) or CsA (50%) and had at least 3 CO measurements during the first year after HT and were included in the study. Between the 4th and 12th month post-HT, the median number of each CNI trough blood concentration levels and the median concentration of those determinations were used to calculate a coefficient of variation (CV) as follows: [CNI trough level standard deviation)/(mean) multiplied by 100]. Patients were divided into high and low CV groups according to the mean CNI-IPV during the first year: 27.8%. Long-term outcomes regarding patient survival and graft loss or rejection were evaluated according to this dichotomization.
The main findings of González-Vílchez et al.12 were: first, patients in the high CV group showed a nonsignificant statistical trend toward a higher risk for the composite of rejection or mortality/graft loss or rejection and all-cause mortality/graft loss 1 to 5 years after transplant (95% confidence interval, 0.993-1.695; P=.056). Second, patients treated with TAC showed a lower CV than those treated with CsA. Third, among the 2 TAC formulations evaluated, extended-release TAC was associated with a lower CV. Finally, in the post-hoc analyses performed in the subset of patients without a history of rejection during the first year (n=967), patients in the high CV group showed a statistically significant increased risk for the composite of rejection, graft loss and 5-year mortality (hazard ratio, 1.609; 95%CI, 1.129-2.295; P=.011). Despite the lack of statistical significance in some of the evaluated items, the statistical trend of the present study agrees with previous data regarding SOT.9,11,13 For instance, in recent renal transplant recipients, an elevated TAC IPV at baseline correlated with the development of donor-specific antibodies5,14 and with an increased risk of allograft rejection. As the authors underscore in the discussion, this could be explained because of the lack of statistical power due to the relatively small number of events in the cohort (138 deaths and 4 retransplants due to graft loss).
As previously mentioned, several factors are implicated in high IPV but nonadherence to the immunosuppressive drug regimen has always been considered the most relevant cause.15 However, no clear evidence has been provided so far. Ko et al.16 have recently been unable to demonstrate a clear relationship between nonadherence to CNI and higher IPV. Nevertheless, in the present study, González-Vílchez et al.12 showed that extended-release TAC showed a lower CV, possibly because of an easier dosing profile and better treatment adherence. Indeed, TAC extended-release formulations have the potential to minimize CO fluctuations,leading to a more stable blood concentration profile.17 On the other hand, a hot topic in SOT is the advent of generic preparations of immunomodulating agents, which constitutes a major challenge for health care providers. Certainly, although considered bioequivalent alternatives, there are well known differences in pharmacokinetic effects between the innovator drug and generics and between the different generic formulations.18 Such important differences are bound to increase IPV and could result in adverse outcomes. Hence, any switch from an branded drug to a generic or between generics should be made only after careful case review and under close monitoring of drug exposure and appropriate patient instructions.
The complex interactions involved in the management of immunosuppression demand a major effort with a multidisciplinary approach to understand the double-sided effects of immunomodulating agents in depth. Measurement of IPV may allow clinicians to early identify at-risk patients and optimize their treatment and follow-up to balance the therapeutic effect and adverse effects of drugs, yet this demands an additional effort from clinicians. Among the potential alternatives for the current standard therapeutic monitoring,the evaluation of the ratio between 2 consecutive CO measurements could help to easily determine IPV and its impact on graft survival.6,19 Initiatives such as that carried out by González-Vílchez et al.12 are crucial to spotlight the importance of IPV in the management of HT patients. Considering the present accumulating data, the clinical community involved in SOT should emphasize the importance of close therapeutic drug monitoring and analysis of the factors involved in variability of blood concentrations of immunomodulating agents. Because the “one dose fits all” rule is imprecise and archaic, the advent of next generation precision medicine regarding pharmacogenetics, biomarkers, big data analyses, new specific devices, artificial intelligence and the internet of things constitutes a strong opportunity to develop new tools for easy, close and patient-centered monitoring, to ensure both graft viability and improvements in patient survival and quality of life.
FUNDINGNone.
CONFLICTS OF INTERESTNone declared.