Introduction
Pulmonary arterial hypertension (PAH) is defined as a group of diseases characterized by a progressive increase in pulmonary vascular resistance caused by vasoconstriction, thrombosis, and structural remodelling of pulmonary arterioles, with occlusion of the lumen of some vessels. If untreated, pressure overload of the right ventricle (RV) leads to progressive RV dilatation and hypertrophy and end-stage heart failure within 2 to 3 years. Conventional therapy with warfarin, digoxin, and diuretics improves symptoms but has a limited effect on disease progression. Calcium antagonists, in selected patients, and prostacyclin analogs significantly improve exercise capacity and prolong survival but treatment is frequently complicated by side effects. Bosentan, an orally active, nonselective endothelin receptor antagonist, has widened the treatment options for PAH it is now an established treatment for PAH patients, often used first line, with prostanoids the first choice for more severely ill patients. Although these recent therapeutic advances have improved the short-to-medium term outcomes for such patients, early death due to progressive RV failure remains inevitable in many.1,2
The diagnostic strategy of PAH requires a series of investigations in order to make the diagnosis, to identify the type of PAH, to evaluate the functional and haemodynamic impairment and to monitor changes in disease status and response to therapy. This diagnostic work-up, in terms of imaging and haemodynamic assessment, includes mainly echocardiography, ventilation/perfusion lung scintigraphy, pulmonary angiography and right heart catheterization.1,3,4 Echocardiography, the most widely used noninvasive technique, is an excellent test for screening and monitoring patients of suspected or known PAH, but has several limitations. Firstly, it relies on geometric assumptions that are difficult to adopt for the complex-shaped RV. The 3-dimensional approach is more promising; however, adequate visualization of the entre RV is not always guaranteed. Secondly, echocardiography depends upon several variables such as detectable tricuspid regurgitation, body habitus, coexisting lung disease, heart rate, posture, and hydration status. (Some of these limitations also affect the accepted gold standard method of right heart catheterization.) Ventilation/perfusion lung scan provides a means for the diagnosis of chronic thromboembolic PAH and its differentiation from idiopathic PAH, but has the disadvantage of exposure to ionizing radiation. Pulmonary angiography is useful in the work-up of chronic thromboembolic PAH and right heart catheterization is required to confirm the diagnosis of PAH, to assess the severity of haemodynamic impairment and to test the vasoreactivity of the pulmonary circulation. However, they are invasive, with exposure to x-rays and (with regard to pulmonary angiography) contrast agents. More recently, spiral computed tomography and cardiovascular magnetic resonance (CMR) have been introduced for cardiac imaging in PAH. Spiral computed tomography is used mainly to assess chronic thromboembolic disease and to rule out acute pulmonary embolism, with the limitation of x-ray exposure. CMR avoids use of ionizing radiation and is increasingly used in patients with PAH for the evaluation of pathological and functional changes of both heart and pulmonary circulation.
Magnetic Resonance in Pulmonary Hypertension
CMR is an attractive modality for studying the complex geometry of the RV since no assumptions need to be made about the shape or location of the structure being studied. CMR has been successfully used as an accurate and reproducible tool to quantify ventricular volumes and mass in both normal and PAH subjects and normal ranges have been established.5,6 Recent technological advances enable the robust implementation of steady-state free precession (SSFP) sequences that provide substantially higher signal-to-noise ratio than can be obtained by conventional gradient-echo techniques, along with excellent contrast between myocardium and blood7 (Figure 1). SSFP technique is rapidly becoming the preferred cardiac CMR pulse sequence for acquisition of volumetric data sets of the left and right ventricles (Figure 2)
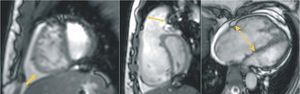
Figure 1. Gradient-echo cine images acquired using the steady-state free precession pulse sequence, from patients with severe pulmonary arterial hypertension. (Left) A short-axis image showing right ventricular enlargement along with increased trabeculation and a D-shaped interventricular septal configuration. Interestingly, this septal flattening remains throughout the cardiac cycle, suggesting both systolic and diastolic right ventricular overload. Note the presence of pericardial effusion (arrow), which is a sign of adverse prognosis in patients with pulmonary hypertension. (Middle) A modified right ventricular outflow tract view from the same patient showing the D-shaped deformity and leftward curvature of the septum. Dilatation of the pulmonary annulus can also be seen (double arrow). (Right) A 4-chamber view demonstrating right-sided cardiac enlargement. Right atrium is prominent and tricuspid annulus is markedly dilated (dashed double arrow).
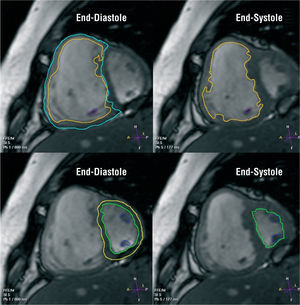
Figure 2. Steady-state free precession cine short-axis images from a patient with severe pulmonary hypertension. Right and left ventricular volumes and mass can be measured from a set of short-axis slices encompassing both ventricles from base to apex. The endocardial borders of the right and left ventricle are manually traced at end- diastole and end-systole. The epicardial borders are traced in end-diastole, for computation of ventricular mass. With the slice thickness known, the volumes contained within all the endocardial borders are summed at each heart phase, and the total volume is then computed. To calculate mass, the volume of myocardium contained within the epicardial and endocardial borders is computed and multiplied by an assumed specific gravity of myocardium (1.05 g/mL). This method of volume measurement is independent of cavity shape and is ideal for the right ventricle.
One of the advantages of CMR is that it provides anatomical measurements and indexes that are not influenced by factors and variables affecting echocardiography. These anatomical indexes are related to haemodynamic parameters clinically relevant with PAH severity, such as the mean pulmonary arterial pressure. The ventricular mass index (ratio of RV mass over left ventricular mass) is such a CMR-derived variable that provides an accurate and practical means of estimating mean pulmonary artery pressure noninvasively, in pulmonary hypertension, and may provide a more accurate estimate than Doppler echocardiography.8 The calculation method of ventricular mass from which this index is derived, is described in Figure 2. Ventricular mass index correlates well (r=0.81) with mean pulmonary artery pressure at right heart catheterisation and its sensitivity and specificity for pulmonary hypertension are 84% and 71% respectively, compared with 89% and 57% of echocardiography.
Moreover, short-axis curvature of the maximal leftward displacement of the interventricular septum in patients with pulmonary hypertension may be used as a marker of pulmonary arterial pressure, as there is a significant correlation between these 2 parameters.9 Septal bowing is evaluated with CMR, measured on short-axis cine images, and expressed as curvature (reciprocal of radius). Curvature is quantified on the image that shows the most severe distortion of normal septal shape. A clear linear relationship between invasive measurements of systolic pulmonary artery pressure and the short-axis curvature of the ventricular septum has been found. A systolic pulmonary artery pressure higher than 67 mm Hg may be expected when leftward curvature is observed.
Another interesting CMR technique for the evaluation of myocardial global and local function is myocardial tagging. Tagged CMR has been established to track the motion of moving tissue by using radiofrequency prepulses that generate gridlines which are tagged onto the image. These taglines move and are deformed as the heart contracts. Myocardial tagging techniques have been used to quantify the three-dimensional global and local deformation, namely the strain patterns, of the RV in normal subjects and open the way for studying alterations in regional RV motion in various types of heart disease.10
In terms of assessment of PAH haemodynamics, phase-shift velocity mapping is a useful CMR approach (Figure 3). In patients with PAH, velocity-encoded CMR provides measurements of pulmonary flow and right-sided cardiac output, similar to those obtained by means of thermodilution, and noninvasive indexes for the assessment of pulmonary vascular resistance, with good reproducibility.11 The curve of pulmonary arterial flow rate as a function of time over the cardiac cycle is used for the calculation of these indexes, namely, the acceleration time (defined as the time from the onset of flow to the peak velocity), the acceleration volume (estimated by integrating the flow rate from the onset of flow to the peak velocity), the maximal change in flow rate during ejection (defined as the maximal value of the ascending slope of the flow rate) and the ratio of maximal change in flow rate to acceleration volume. Inverse correlations have been found between pulmonary vascular resistance and acceleration time or acceleration volume. Pulmonary vascular resistance is directly correlated (r=0.89) with the ratio of maximal change in flow rate to acceleration volume. This ratio can be used to distinguish patients with high pulmonary vascular resistance (≥67 mm Hg/min/L-1) from patients with near normal pulmonary vascular resistance or healthy subjects.
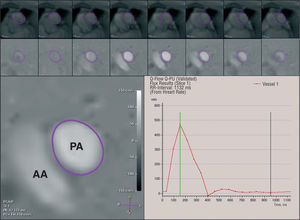
Figure 3. Phase-shift velocity mapping in a plane transecting the pulmonary trunk. The principle underlying this technique is based on the phase-shift of the moving protons which is proportional to blood flow velocity. On the resultant images, pixel values are linearly related to velocity (bottom left). For pulmonary flow measurement, the cross-sectional area of the pulmonary trunk lumen is outlined manually in each cine frame throughout the cardiac cycle, and the mean axially directed velocity is measured within that area (top and middle row). The respective instantaneous flows are then derived from the products of the individual luminal areas and the mean velocities within them. From these data, pulmonary flow-time course curves can be generated (bottom right). PA indicates pulmonary artery; AA, ascending aorta.
Another computed method which relies on a mathematical technique enables the noninvasive assessment of mean pulmonary arterial pressure from phase-shift velocity mapping by computing both physical and biophysical parameters.12 The physical parameters include the mean blood flow velocity over the cross-sectional area of the main pulmonary artery at the systolic peak and the maximal systolic value of the cross-sectional area of the main pulmonary artery (obtained by velocity-encoded CMR), whereas the biophysical parameters are related to each patient, such as height, weight, and heart rate. These parameters have been measured in patients undergoing right-side heart catheterization, and a significant correlation was found between the computed mean pulmonary arterial pressure value and the mean pressure value obtained from catheterization.
Pulmonary vascular and RV function often determine morbidity and mortality in patients with primary or persistent forms of secondary PAH and their precise monitoring is essential for optimal decision making. Therefore, CMR-derived anatomical and haemodynamic parameters could be helpful to screen patients with PAH to decide whether catheterization should be performed and for the follow-up of heamodynamic changes associated with medical therapies, such as long-term vasodilator treatment.2
One of the most significant advances in CMR has been the ability to directly visualize the non viable myocardium by using a segmented inversion-recovery gradient-echo sequence after the intravenous administration of gadolinium contrast. With this technique, termed delayed contrast-enhanced CMR, irreversibly damaged myocardium (fibrosis) is depicted as hyperenhanced (bright).13 Recently, it was shown that delayed contrast enhancement was present within the RV insertion points and interventricular septum of most patients with PAH.14 Septal contrast enhancement was associated with interventricular septal bowing. The extent of contrast enhancement correlated positively with RV end-diastolic volume/body surface area, RV mass, mean pulmonary arterial pressure and pulmonary vascular resistance and correlated inversely with RV ejection fraction. Delayed contrast-enhanced CMR may provide a novel marker for occult septal abnormalities directly relating to the haemodynamic stress experienced by patients with PAH. Given the correlations of contrast enhancement with existing markers of disease severity in PAH, this parameter may prove useful in the prognostic classification of these patients.
In terms of evaluation of the pulmonary circulation, gadolinium contrast-enhanced, three-dimensional magnetic resonance (MR) pulmonary angiography is a promising modality for the identification of patients with PAH. A right pulmonary artery diameter value >28 mm is sensitive (89%) and highly specific (100%) for the diagnosis of chronic PAH.15 Especially in chronic thromboembolic PAH, MR angiography seems to have great potential in detection of vascular changes (central thromboembolic material, vessel cut-offs, and abnormal proximal-to-distal tapering) rather than in other causes of PAH4 (Figure 4). Although the combination of right heart catheterization and selective pulmonary digital subtraction angiography is still regarded as the standard of reference with respect to establishing the diagnosis, assessing the severity and determining the technical feasibility of thromboendarterectomy for chronic thromboembolic PAH, contrast-enhanced MR angiography depicts the characteristic angiographic findings in all patients, and its results show very good correlation with those of digital subtraction angiography. After surgery, MR angiography enables documentation of the procedural success and it may play (in combination with functional and flow measurements) an important role in postoperative follow-up.16
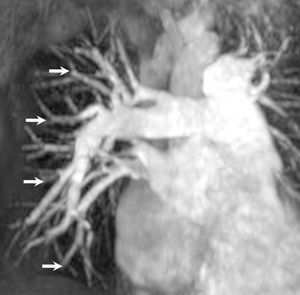
Figure 4. Gadolinium contrast-enhanced magnetic resonance angiography of pulmonary vasculature in a patient with primary pulmonary hypertension. Spatial impression on this image has been obtained by means of a three-dimensional data reconstruction method termed "maximum intensity projection." The image demonstrates large central pulmonary arteries and diffuse reduction of smaller peripheral vessels (arrows), with no evidence of thrombotic material in the pulmonary artery tree.
In order to evaluate pulmonary perfusion, two approaches can be used.17 The first approach implements contrast-enhanced MR imaging techniques using two-or three-dimensional ultrafast imaging sequences. Multiple images are acquired rapidly during a bolus intravenous administration of a gadoliniumbased contrast agent. Acquisition rates of up to 3 images per second have been used. Regions of decreased parenchymal blood flow will be identified as areas of poor regional contrast enhancement. The second approach to pulmonary perfusion imaging uses sequences that do not require intravenous contrast agents. These techniques are known as arterial spin labeling, and they use the protons within intravascular blood as an endogenous tracer for the evaluation of blood flow. While MR perfusion techniques are able to document changes in blood flow with pulmonary hypertension, they are not commonly used at present and their importance in the diagnosis and management of this disease is not known.
In terms of magnetic resonance imaging of the lung per se, this is inherently difficult due to the low proton density and the large number of soft tissue-air interfaces, both of which reduce the amount of emitted signal. However, advances in hardware design and imaging sequences have led to improved evaluation of the lung parenchyma.4
Recent improvements in hardware and software have enabled cardiac catheteriation under real-time CMR imaging (MR fluoroscopy). MR fluoroscopy allows the guidance of a balloon tipped catheter in the right atrium, the RV outflow tract and the main pulmonary artery. The balloon is inflated with carbon dioxide and produces a circumscribed susceptibility artefact which is clearly distinguished from the bright blood pool of the heart and main pulmonary artery and allows the visualization of the catheter's tip. Recent works have demonstrated the feasibility of combining invasive RV and pulmonary artery pressure measurements, under MR fluoroscopy, and velocity-encoded CMR derived flow data to quantify pulmonary vascular resistance in humans.18,19 This novel method which has been validated against thermodilution technique in animal models and proved advantageous over the Fick principle, raises the possibility of a more accurate method of pulmonary vascular resistance quantification, leading to better treatment of patients with PAH.19,20
CONCLUSIONS
In conclusion, CMR is a promising and versatile modality that should be considered as a complementary diagnostic tool for the evaluation of patients with PAH. Although right heart catheterization remains the gold standard for the assessment of pulmonary haemodynamics and echocardiography rules the routine clinical practice, the unique features of CMR render it ideal for the noninvasive monitoring of pulmonary vascular and RV function in PAH. Therefore, it can possibly play an important clinical role in guiding management and determining prognosis in this patient population. Nevertheless, additional experience is needed before introducing this tool in the routine assessment of pulmonary hypertension.
Correspondence: Petros Nihoyannopoulos, MD, FRCP, FACC, FESC,
National Heart & Lung Institute, Imperial College Faculty of Medicine,
Hammersmith Hospital, Cardiology Department,
Du Cane Road, London W12 0NN, UK,
Tel: +(0)20-8383 3948,
Fax: +(0)20-8383 4392,
E-mail: petros@ic.ac.uk