Although bifurcation lesions account for 15% to 20% of percutaneous interventions (PCI), their optimal management is still debated.1 Such lesions pose a technical challenge for PCI and are associated with both higher rates of periprocedural complications and subsequent stent failure. Although second generation drug-eluting stents (DES) have reduced rates of restenosis after bifurcation PCI compared with earlier generation DES,2 significant challenges remain. These include the avoidance of abrupt side branch (SB) closure, mitigation of the higher risk of stent thrombosis compared with nonbifurcation lesions, and prevention of restenosis, especially at the SB ostium. Intervention with bioresorbable vascular scaffolds (BVS) might potentially address some of the limitations of conventional metallic DES in this setting.3
To date, 2 drug-eluting BVS have received CE mark approval for use in Europe. Both are based on scaffolds constructed from lactic acid polymers: the everolimus-eluting ABSORB stent (Abbott Vascular, Santa Clara, California) and the novolimus-eluting DESolve stent (Elixir Medical Corporation, Sunnyvale, California). Based on encouraging clinical trial results, a third device composed of a magnesium backbone may receive CE mark approval later this year.4 In the United States, no devices are currently approved for use, though a Food and Drug Administration advisory panel recently supported a premarket approval application for the ABSORB BVS system in March 2016.5
Bioresorbable vascular scaffolds work by providing temporary scaffolding to prevent acute vessel closure or recoil in addition to transient drug elution to prevent neointimal hyperplasia, before fully degrading (over a period of 2-4 years in the case of lactic acid-based devices).6 In some respects, there is reason to believe that the theoretical advantages of BVS over metallic DES implantation may be more pronounced in bifurcation lesions. First, as the degree of delayed arterial healing seen with conventional DES may be higher after bifurcation intervention, especially if a dual stent technique is used,7 the benefit for the patient of a disappearing stent may be greater. Second, late luminal enlargement–due to positive vessel remodelling as the scaffold degrades–may be particularly beneficial at bifurcation sites, given the increased risk of restenosis at these sites compared with nonbifurcation sites. Third, in a bifurcation lesion where the main vessel is stented, long-term jailing of the SB may be avoided following BVS resorption.
Nonetheless, a number of limitations associated with the use of this technology at bifurcation sites must equally be considered. Due to their polymeric nature, BVS have different structural and mechanical properties to metallic stents.3 First, they require thicker and wider struts than metallic stents to provide adequate radial strength for vessel scaffolding. Per manufacturer reported measurements, the ABSORB and DESolve scaffolds have strut thicknesses of 157μm and 150μm, respectively, compared with 89μm for the Xience metallic DES (Abbott Vascular), for example. Greater strut width translates into larger device footprints of 27% and 30% respectively, compared with 13% for Xience (for 3.0mm devices deployed at nominal pressure in each case).8 This has a number of implications: BVS are bulky–with a crossing profile of ∼1.4mm for ABSORB and DESolve, compared with ∼1.1mm for Xience8–which may hinder device delivery in a bifurcation lesion or passage through the struts of a deployed stent or scaffold during a bifurcation PCI. In addition, thick struts are more thrombogenic than thinner struts on account of more turbulent blood flow and slower endothelialisation.9 This risk is amplified in a dual-stent technique, when there may be overlap of 2 to 3 stent layers at the carina. Furthermore, in situations where the SB is jailed, wider struts may actually increase the risk of acute SB occlusion and periprocedural myocardial infarction on account of their larger footprint.10 Moreover, formation of a neointimal bridge on jailed ABSORB BVS struts prior to scaffold resorption has been reported, with a consequent reduction in SB ostial flow area.11 Second, polymeric struts break more easily than metallic struts, which limits their expansion capacity. This places restrictions on postdilatation techniques–such as proximal optimization or kissing balloon angioplasty–both of which are important components of contemporary bifurcation PCI. Third, BVS are more cumbersome to implant than metallic DES, requiring more time for lesion preparation, device delivery, prolonged inflation and postdilatation. This results in a significant increase in procedure time compared with conventional DES–an issue that is amplified in the setting of bifurcation PCI.
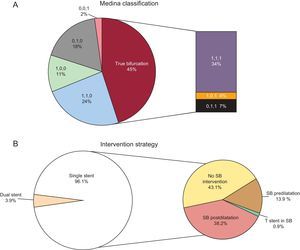
In a recently published article in Revista Española de Cardiología, Suárez de Lezo et al12 report data regarding 230 coronary bifurcation lesions treated with BVS in a single center registry.12 Patients had clinical follow-up at a mean of 14 months. The main finding of the report was that in carefully selected patients with suitable lesions, procedural success rate was high, midterm adverse imaging outcomes were favorable, and rates of adverse clinical events were low. The authors have great experience in bifurcation PCI and should be congratulated for reporting a large dataset of bifurcation lesions treated with BVS. A number of important strengths should be acknowledged. First, true bifurcation lesions–with significant disease in both the main vessel and SB–were present in a considerable proportion of cases–45% overall (Figure, panel A). However, due to a pragmatic approach to intervention, procedural success was achieved with a single-stent strategy in 96% of lesions, with a very low rate of dual stenting required (Figure, panel B). Second, intravascular imaging at the time of PCI was liberally used–either intravascular ultrasound in 60 lesions or optical coherence tomography in 87 lesions. This included 90% of those that underwent SB dilation through the cells of the BVS. Moreover, in all patients treated with a modified sequential kissing balloon angioplasty technique, the intervention was guided by intravascular ultrasound. Third, 78% of lesions were evaluated with angiographic surveillance at a mean of 7.3 months. Impressively, at angiographic follow-up, all SB were patent. A total of 12 (5%) patients showed restenosis and required repeat revasularization. The most common site was at the proximal edge of the BVS, with no differences in restenosis rates between lesions in which the SB was postdilated and those that were not.
Distribution of lesions in the article of Suárez de Lezo et al.12 according to the Medina classification (A) and treatment strategy used (B). SB, side branch.
This study also has a number of important limitations which must be considered when interpreting the results. First, its single-arm design precludes comparison of BVS implantation in bifurcation lesions with other potential therapies. Moreover, a single specialized center experience limits the external validity of the results. Indeed, whether these outcomes are generalizabile to routine practice in centers with lesser expertise is an open question. Second, the use of computed tomography angiography rather than invasive angiography in most of the patients with imaging surveillance is another limitation, given that computed tomography is not validated for this indication. Third, the duration of follow-up was limited. Given that it takes up to 4 years for the ABSORB BVS to fully resorb,6 the natural history of the device in these lesions is not fully captured within the time frame of the current study.
Finally, despite the encouraging results reported, the implantation protocol used was not consistent with contemporary standards. In particular, lesion predilatation was performed in less than half of lesions in the current report. Lesion predilatation was mandated in the clinical trials of ABSORB BVS13–16 and was performed in the overwhelming majority of patients enrolled in recent registries.17–19 Furthermore, due to low strut penetrance compared with metallic stents and lower radial strength, high pressure postdilatation of BVS is increasingly regarded as standard-of-care. However, in the present report, only about half of patients were treated with postdilation.
In view of the above, how should we interpret the current results in bifurcation lesions against the background of recently published randomized clinical trials? In our opinion, 3 central messages have emerged. First, in selected patients enrolled in carefully supervised randomized trials, overall clinical outcomes at around 1 year–as measured by composite endpoints such as target lesion failure–are broadly comparable when BVS are compared against everolimus-eluting metallic stents.20 Second, in studies that included angiographic surveillance, BVS showed a marginally inferior performance compared with the same comparator. However, the magnitude of this difference is small–a mean difference of the order of 0.08 mm–and of low clinical relevance. Third, the rate of stent thrombosis with BVS is about twice as high in comparison with everolimus eluting metallic stents. The reasons for this are manifold and include the risk related to thicker and less penetrative stent struts, as well as the increased challenges associated with deployment. In this respect, the efforts of the investigators in the current report are noteworthy: 3 patients had definite or probable ST–2 subacute and 1 late–with an incidence of 0.87% at 30 days and 1.3% at 12 months. This is low compared with other registry data. Although this rate needs to be interpreted with caution for the reasons already discussed, it might be related to the high rate of intravascular imaging use in this study. Interestingy, both cases of definite ST occurred in the setting of deficiencies in antithrombotic therapy, with 1 case of subacute ST occurring in a clopidogrel-resistant patient and 1 case of late ST in a patient who was nonadherent to aspirin and clopidogrel therapy. This reinforces the paradigm that stent thrombosis is usually multifactorial in etiology and manifests typically when a number of adverse clinical features align simultaneously. Devices that are somewhat more thrombogenic are less forgiving when deficiencies exist in relation to other clinical factors such as platelet inhibition. In fact, a case could be made for prescribing more potent dual antiplatelet therapy after BVS implantation, at least in the initial months poststenting.21 This strategy would of course impact on bleeding risk and has not been validated in a randomized trial.
In conclusion, the current report demonstrates that in expert hands, BVS implantation can be performed safely in coronary bifurcation lesions, when intravascular imaging guidance is used and BVS expansion limits are respected. In a time when BVS use is increasing in some jurisdictions, selection of the correct patient, lesion, and scaffold implantation technique, and liberal use of adjunctive intravascular imaging are critical components of BVS success. However, although the observations of Suárez de Lezo et al are encouraging, the benefits to the patient over the long-term remain ill-defined. The results of long-term follow-up from ongoing large-scale trials comparing BVS with conventional DES are eagerly awaited. Only then will we appreciate whether the additional challenges and costs associated with BVS implantation translate into tangible clinical benefit for our patients.
CONFLICTS OF INTERESTR.A. Byrne reports lecture fees from B. Braun Melsungen AG, Biotronik and Boston Scientific and research grants to the institution from Boston Scientific and Heartflow.