2009. Where are we in atrial fibrillation ablation? In the last few years, new horizons in atrial fibrillation (AF) ablation came closer became more visible as this approach has become the curative treatment modality for several tachyarrhythmias in many electrophysiology laboratories worldwide. Just over a decade ago, Haissaguerre et al and Pappone et al1-6 firstl demonstrated the potential therapeutic role of catheter ablation to treat patients with AF. These preliminary observations ushered in the modern era of catheter ablation to treat patients with AF, and considerable progress has been made in understanding the physiopathology of arrhythmias as well as different catheter ablation strategies to treat AF. There is evidence to show that wide area circumferential ablation, as initially proposed by Pappone et al2-6, may result in lower rates of AF recurrence than ostial pulmonary vein isolation in patients with either paroxysmal or persistent AF.7 In the last 5 years, several randomized studies from different centers worldwide provided evidence that patients with AF who received catheter ablation as a second line therapy (ie, in patients who did not respond to antiarrhythmic drug therapy [ADT]) had a higher probability of maintaining sinus rhythm compared to those treated with ADT alone at 12 months.7-11 Unlike paroxysmal AF, currently, less consensus exists as to the most appropriate ablation strategy to treat patients with persistent, long-standing or permanent AF.12,13 In fact, as AF progresses from the paroxysmal form to the persistent or permanent forms, electrophysiologic and structural changes develop over time, resulting in marked extracellular fibrosis, which in turn strongly potentiates, atrial myocardial substrate for re-entry.12 During such progression, the role of focal triggers significantly decreases while the importance of substrate and re-entry significantly increases. As a result, in patients with nonparoxysmal AF (persistent/permanent AF) the efficacy of pulmonary vein (PV) isolation alone is greatly reduced and, as firstly suggested by Pappone et al,1,3,4 linear lesions within the left atrium are frequently required in a step-wise fashion, particularly in the interatrial septum, the base of the left atrial appendage, and/ or the inferior left atrium along the coronary sinus. During the step-wise catheter ablation strategy, the cardiac rhythm often converts from AF to organized macroreentrant or microreentrant atrial tachycardias which can be targeted for ablation. Although feasible, this approach is limited by longer procedure times, often requiring several hours, with postablation atrial tachycardia recurrence necessitating redo ablation procedures. Therefore, further technical and scientific advances are required in patients with persistent long-standing or permanent AF to overcome these important limitations while refining the best ablation approach in terms of both safety and efficacy. If performed in experienced centers by experienced operators, catheter ablation of both paroxysmal and persistent AF is a safe procedure, although complications may occur, particularly in elderly patients with longstanding AF and enlarged atria. The most important are PV stenosis, thromboembolism and stroke, perforation with cardiac tamponade, phrenic nerve injury, and atrioesophageal fistula. Indeed, it is now well established that if too much radiofrequency energy is applied within a PV, stenosis can occur. Although this complication was common early in the ablation experience, symptomatic PV stenosis is now uncommon, with a frequency of approximately 1%. This decreased incidence is partly a result of the more careful use of various imaging modalities (eg, computed tomography and/or magnetic resonance imaging [MRI], electroanatomic CARTO/or EnSite systems, and intracardiac ultrasound) to prevent inadvertent ablation deep within a PV. Consistent with the recent ThermoCool data (unpublished observations), serious complications associated with radiofrequency catheter ablation are indeed uncommon, indicating that safety of catheter ablation procedures as now practiced is reassuring. Currently, with a complication rate of about 5%, mostly vascular in nature, absence of pulmonary vein stenosis, atrioesophageal fistulae and embolic events including stroke, atrial fibrillation ablation can be performed with an acceptable complication rate for a progressive long-term disease. Despite these good data, any further procedure safety improvement remains an important area of interest.
The View Beyond 2009: Remote-Controlled Atrial Fibrillation Ablation
The expansion of indications for catheter ablation from supraventricular tachycardias to complex tachyarrhythmias such as AF leads electrophysiologists to face prolonged procedure times, excessive fluoroscopy exposure and the need for stable and reproducible catheter movement, all of which can be overcome by remote-controlled ablation systems.14,15 Based on the improved efficacy and safety of anatomical PV isolation as initially proposed by Pappone et al,2-6 several approaches have been advanced to achieve this electrophysiologic end point. Indeed, during early procedures targeting PV isolation alone as first suggested by Haissaguerre et al,1 operators frequently spent many hours with multiple catheters positioned in PVs waiting for AF-initiating premature ectopic depolarizations to occur. Beyond prolonged procedure duration, the first approach proposed by the Bordeaux group was frequently followed by clinical recurrences related to new foci which were not elicited at the index procedure. Circumferential anatomical PV isolation also has one very important safety advantage compared with focal ablation of AF triggers: avoiding PV stenosis, a dreaded complication that has a strong tendency to recur as restenosis after balloon venoplasty. If the circumferential isolating ablation lesion set is placed outside the PVs, the risk for stenosis is minimized. This review reflects on some of the major unanswered questions about AF management, and the future technological and investigational directions being explored in the nonpharmacological management of AF. The difference in clinical outcome after ablation of paroxysmal AF is very likely related directly to the ability of the operator to manipulate and stabilize the position of the ablation catheter with the requisite force to generate effective point-by-point ablation lesions. To improve the technical feasibility of the procedure and thereby the continuity of the isolating ablation lesion sets, extensive effort has been made to improve the ablation technology. Remote-controlled navigation technology provides for precise navigation with the hope that this translates to improved lesion contiguity. Recently, remote-controlled robotic catheter ablation has emerged as a new ablation approach to achieve these goals.14,15 Two remote-controlled systems, wich have the added benefit of reducing the physician's radiation exposure, have been developed to facilitate catheter navigation and ablation by increasing catheter stability. The first one available for clinical use is a magnetic navigation system (Niobe II system, Stereotaxis, St. Louis, Missouri) and the second one is a robotic navigation system (Sensei system, Hansen Medical, Mountain View, California). Although both systems have shown the feasibility and safety of remote-controlled ablation, further technological innovations are required to expand applicability and research is needed to establish non-inferiority to manual approaches. Therefore, technical innovations are clearly warranted which could: a) minimize the physician's fluoroscopy exposure; b) reduce physical demands on the operator by allowing for a more relaxed ablation procedure from within the control room; c) improve catheter stability and reproducibility of the procedure; and d) increase patients' safety by avoiding serious complications.
Remote Magnetic Navigation
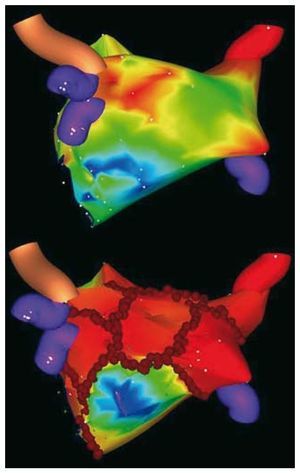
The Stereotaxis navigation system includes two large external magnets positioned on either side of the fluoroscopy table to generate a uniform magnetic field (0.08 T) of approximately 15-cm diameter within the patient's chest.14,15 The ablation catheters are extremely floppy along their distal end with small magnets embedded at the tip of the catheter. The catheter tip aligns with the orientation of the generated magnetic field. Using a software interface, the operator can manipulate the magnetic field and, by extension, the tip of the ablation catheter, providing the first level of freedom of movement with this new system. The other level of freedom of movement is the ability remotely to advance or retract the catheter tip by a computer-controlled catheter advancer system (CardioDrive), which consists of a disposable plastic unit positioned at the femoral catheter insertion site. The catheter shaft is affixed to a CardioDrive unit where it enters the sheath, and can transduce the remote operator instructions to advance or retract the catheter appropriately. This combination of remote catheter advancement-retraction and magnetic field manipulation allows the operator a great deal of flexibility for navigation, mapping and ablation. The magnetic navigation system is integrated with one of the electroanatomic mapping systems (CARTO RMT, Biosense Webster, Diamond Bar, CA), which also allows integration of 3-dimensional computed tomography or MRI models. Once integrated, the magnetic field can be directly controlled with the computer mouse. The mapping system can precisely localize the catheter tip in space to a submillimeter resolution. By precisely tracking the catheter location, the combination of mapping and navigation systems allows automated chamber mapping. The operator can remotely manipulate the magnetic catheter within the left atrium to a predefined anatomic location, such as PV ostia or mitral valve annulus. Based on these parameters, the system automatically manipulates the catheter throughout the chamber to facilitate generation of an electroanatomic map. New software allows the system to manipulate the catheter tip automatically to create linear ablation lesions with in the chamber as per the operator's wishes. The efficiency and accuracy of these automatic software solutions, however, remain to be demonstrated. The other significant advance is the ability to incorporate preacquired 3-dimensional MRIs or computed tomography scans into the system to allow mapping on a realistic model of the heart. With the current generation software, clinical data are available on its efficacy for AF ablation. In a consecutive series of 40 patients, magnetic mapping and navigation systems were used in tandem to assess the feasibility and safety of circumferential PV ablation in patients undergoing catheter ablation of AF. In our preliminary experience, using a 4-mm ablation catheter tip (with the requisite embedded magnets), the left atrium and PVs were successfully mapped in 38 of 40 patients.15 Ablation lesions were placed in a circumferential fashion for a maximum of 15 seconds at any endocardial ablation site; procedure times decreased significantly with increased operator experience. The end point was >90% reduction in the bipolar electrogram amplitude, and/or peak-to-peak bipolar electrogram amplitude <0.1 mV inside the line. Magnetically enabled irrigated ablation catheters with the requisite embedded magnets to permit remote navigation have been recently used for the first time in our laboratory. In more than 100 patients with AF, the results have demonstrated that electroanatomic maps are accurate and that the standard set of lesions with remote PV isolation can be reproducibly achieved using an irrigated ablation catheter (Figure).
Figure. Non-fluoroscopic mapping system images created using a new magnetic irrigated-tip catheter. Postero-anterior view of the left atrial geometry before (top panel) and after circumferential pulmonary vein ablation (CPVA) with ablation lesions in red (bottom panel). Red circles indicate the standard set of CPVA lesions around pulmonary veins and additional linear lesions (mitral isthmus, roof and posterior lines).
Remote Robotic Navigation
The remote navigation capability of robotic systems (Sensei) is based on multiple pullwires that control the deflection capability of 2 steerable sheaths. This "master-slave" electromechanical system controls an internal steerable guide sheath and an external steerable sheath. The internal sheath contains four pullwires located at each quadrant; the range of motion includes 360-degree deflection and the ability to insert and withdraw. The external sheath has a single pullwire to allow for deflection, and can rotate and insert and withdraw, permitting a wide range of motion in any direction. Unlike the Stereotaxis system, standard ablation catheters can be used with this remote robotic system because the inner steerable sheath can accommodate any type of catheter up to 8.3-French diameter. By fixing the mapping-ablation catheter so that it is just protruding beyond the tip of the inner system, remotely driving these steerable sheaths results in remote navigation of the catheter tip. The steerable sheaths are attached to the remote robotic arm unit, which can be mounted to any standard radiography procedure table. Using a software interface, a 3-dimensional joystick allows the operator to drive the catheter tip. Movements of the joystick are translated into a complex series of manipulations by the pullwires governing sheath motion. The long-term safety and efficacy of PV isolation using this robotic navigation system remains to be established.
Balloon-Based Pulmonary Vein Isolation
Balloon ablation catheter technology uses various ablation energy sources designed to rapidly isolate the PVs more simply than standard manual point-by-point radiofrequency applications.16 Analysis of 3-dimensional left atrial-PV surface reconstructions from MRI on patients with paroxysmal AF showed a marked intrapatient and interpatient variability in PV ostial size and geometry.16 The challenge to each of the balloon ablation catheters is to negotiate this venous anatomy so that the lesions are proximal enough to include all of the potentially arrhythmogenic periostial tissue while minimizing the risk of PV stenosis. The energy source used also has important implications in terms of safety, and cryothermal ablation has minimal risk for PV stenosis. A balloon cryoablation catheter may be used safely even deep within large common PVs. The adjustable catheter design of the endoscopic balloon catheter, however, allows the operator to vary the circumference and location of the ablative beam. This is extremely useful in patients who have veins with marked variability in size and shape. Alternatively, because high-intensity focused ultrasound energy can be delivered through blood with minimal risk, this energy modality might be efficacious in isolating large PV ostial or antral regions through delivering a series of sequential lesions as it is precessed about the long axis of the targeted vein. Preliminary observational studies have reported that many patients with paroxysmal AF can be treated successfully with balloon technology. Further randomized studies will determine whether all or any balloon technology may create safe, reproducibly effective PV isolation with good long-term outcome.
Future Directions
New data show improved long-term outcomes among patients with AF by challenging the belief that progression of AF to more persistent forms is inexorable and irreversible. Our recent experience in patients with new onset AF indicates for the first time that in the natural history of AF, early rhythm control by catheter ablation at the time of paroxysmal AF, may limit rapid progression to persistent/permanent AF, particularly in patients with associated diseases.17
Correspondence: C. Pappone, MD,
Department of Electrophysiology and Cardiac Pacing, San Raffaele University Hospital,
Via Olgettina, 60, 20132, Milan, Italy
E-mail: carlo.pappone@hsr.it