Atrial fibrillation is the commonest cardiac arrhythmia, with significant morbidity related to symptoms, heart failure, and thromboembolism, which is associated with excess mortality. Over the past 10 years, many centers worldwide have reported high success rates and few complications after a single ablation procedure in patients with paroxysmal atrial fibrillation. Recent studies indicate a short-term and long-term superiority of catheter ablation as compared with conventional antiarrhythmic drug therapy in terms of arrhythmia recurrence, quality of life, and arrhythmia progression. As a result, catheter ablation is evolving to a front-line therapy in many patients with atrial fibrillation. However, in patients with persistent long-standing atrial fibrillation catheter ablation strategy is more complex and time-consuming, frequently requiring repeat procedures to achieve success rates as high as in paroxysmal atrial fibrillation. In the near future, however, with growing experience and evolving technology, catheter ablation of atrial fibrillation may be extended also to patients with long-standing atrial fibrillation.
Keywords
.
IntroductionAtrial fibrillation (AF) is the commonest cardiac arrhythmia and carries with it significant morbidity and mortality, specifically increasing risk of stroke and heart failure.1 In addition, AF naturally progresses towards persistent AF at an estimated rate of 15% to 30% over a 1- to -3-year period2, 3, 4, 5 with increased risks and complications. It is well known that AF doubles the possibility of death, mainly due to the higher incidence of thromboembolic events and occurrence of heart failure. The most important treatment objectives are to avoid such serious arrhythmia complications and progression while improving the quality of life by stable sinus rhythm maintenance. Although antiarrhythmic drugs (AAD) may affect the electrical activity of the heart, avoiding initiation and perpetuation of the arrhythmia, it has been reported that AAD (alone or in combination) may be effective in the long term in only a minority of patients with AF. In addition, chronic administration is required to maintain a stable sinus rhythm, which is often associated with intolerable or serious side-effects requiring AAD withdrawal. Catheter ablation of AF was proposed many years ago as an alternative to long-term AAD therapy, but AF ablation is a relatively new procedure and the very long-term outcomes are not known. A large, prospective, nonrandomized follow-up study of patients with both paroxysmal and chronic AF reported by our group demonstrated for the first time a long-term benefit of catheter ablation in terms of arrhythmia recurrence, morbidity, and mortality as compared with AAD.6 In the last few years, the results of randomized studies have demonstrated a short-term superiority of catheter ablation as compared with conventional AAD in the treatment of patients with paroxysmal or chronic AF.7, 8, 9, 10, 11, 12, 13, 14 Following these encouraging data, catheter ablation as class I with level of evidence A has now been recommended for patients with symptomatic paroxysmal AF and normal or mildly dilated left atrium (LA) and normal or mildly reduced left ventricular ejection fraction1 but long-term data to substantiate this recommendation are limited. A more recently published randomized study from our group15 reports the final results of the APAF study,14 which compared AAD and radiofrequency (RF) ablation (RFA) in 198 patients initially randomized to catheter ablation (98 patients) or to AADs (98 patients). The results at 4 years after randomization demonstrated for the first time that catheter ablation is more effective than chronic AADs with flecainide, sotalol, or amiodarone (alone or in combination) in many patients initially randomized to catheter ablation with repeat interventions in about one third of them.15 By contrast, conventional AAD therapy was ineffective or associated with serious cardiac effects in almost all patients, frequently requiring crossover to RFA for recurrent arrhythmia and associated complications or arrhythmia progression.15 Therefore, these new data further support recent recommendations, as suggested by current guidelines,1 since catheter ablation strategy with repeat interventions as necessary, rather than chronic AADs, may provide long-term benefits in terms of recurrent arrhythmia, complications, and quality of life. The strategy of chronic conventional AAD therapy at standard dosages and switching to catheter ablation only if the patient does not have a response is less effective than the strategy of performing RFA initially.15, 16, 17, 18 In the APAF study the patients randomized to RFA had a significant long-term improvement of quality of life scores at the end of the study while almost all patients initially randomized to AADs and before crossing to catheter ablation showed a poorer quality of life, which improved only after crossover to RFA.15 Therefore, in contrast to rate control or AADs, catheter ablation offers the possibility of a lasting cure and now it seems appropriate to expand its indications. With time and operator experience, catheter ablation of AF has proved to be a very effective strategy for AF, with similar success rates being reported by several different groups.
Catheter Ablation Strategy in Atrial FibrillationCurrently, catheter ablation is one of the fastest-growing techniques in cardiology and due to the very high number of patients with AF that might be candidates to this procedure, a significant number of resources will have to be devoted to it to be able to treat them in coming years. The optimal ablation strategy which can be successfully applied to all forms of AF ranging from paroxysmal to permanent is not yet well defined. Pulmonary vein (PV) isolation (PVI) or circumferential pulmonary vein ablation (CPVA) have emerged as an optimal alternative to chronic AAD for patients with paroxysmal AF since a significant number of AF episodes initiate in the PV area.19, 20, 21, 22 The effectiveness of these techniques in these patients is around 70% to 90%, but in about 10% to 25% a second procedure is required. The overwhelming majority of individuals undergoing AF ablation will not experience a complication. However, as with any invasive procedure, complications can occur.23 Potential minor complications including bleeding or bruising at the catheter sites occur rarely (1%-2%). Major complications such as a stroke, heart attack, or puncturing the heart, lung, or esophagus occur in less than 1% of patients. The risk of blocked pulmonary veins was a greater concern only with PVI alone, as initially proposed by the Bordeaux group, but CPVA as initially proposed by our group and the current technique for PVI minimizes this risk. In case of success the patient does not require continuation with AAD and the arrhythmia is definitively cured. Some individuals, however, may require antiarrhythmic medications after the procedure to prevent AF recurrences. Medications that were previously ineffective prior to an AF ablation may become much more effective after ablation. Options for highly symptomatic patients who do not respond to AF ablation include rate control medications, or an AV node ablation and pacemaker. It is estimated that many thousands of AF ablation procedures are performed annually in Europe and the number has been increasing exponentially in recent years; availability of more sophisticated techniques/equipments has produced a marked increase in the number of centers performing AF ablation. Many patients are asymptomatic but seek catheter ablation as an alternative to long-term anticoagulation with warfarin. Three-dimensional (3-D) mapping systems, robotic techniques, new energy sources, and new, more reliable catheters are easing the ablative procedure and improving efficacy and safety.20, 21, 22 An optimal ablation strategy as a permanent cure for persistent AF requires identification and elimination of all arrhythmogenic sources and substrates for AF termination. Therefore, the approach for these patients, who at present are increasingly referred for catheter ablation, is still evolving.24 The presence of fibrosis and markedly dilated LA is associated with a lower probability of a successful outcome. Therefore, in such a patient population the risks and benefits of the procedure should be individually evaluated. The initial excellent results obtained in patients with lone paroxysmal AF or in patients with minimal structural heart disease encouraged many electrophysiologists to include patients with chronic AF, but poorer long-term results were reported using PVI alone24 while better results were obtained with CPVA that includes additional linear lesions in the LA after PVI.8 What became clear was that catheter ablation of PV triggers alone is unable to maintain a stable sinus rhythm in many patients with persistent arrhythmia in whom areas of slow conduction allow transformation of ectopic activity into multiple reentrant circuits and continuous fibrillatory activity.24 For these reasons, additional linear ablation as initially performed in CPVA, has become a valid strategy in the treatment of patients with both paroxysmal and persistent AF, and is increasingly performed worldwide to treat patients with AF. Since 1999 we have used linear ablation strategy guided by 3-D mapping systems to reduce the amount of tissue available to sustain reentry in patients with both paroxysmal and chronic AF.20, 21, 25, 26, 27, 28 By contrast, failure to eliminate paroxysmal AF in many patients and the impressive number of recurrences in persistent AF after PVI alone confirmed that substrate reduction (or “atrial debulking”) by linear ablation should be considered as an important end point for AF elimination even in patients with paroxysmal AF, as first hypothized by our group.20, 21 In our experience, successful linear ablation requires an extensive use of 3-D mapping systems which can integrate anatomic and electrophysiologic information to avoid potential gaps, thus minimizing complications such as post-ablation atrial tachycardias (AT).28 In patients with paroxysmal AF and minimal or no structural heart disease, who represent only a large minority of patients with AF, multiple linear lesions in the LA may be unnecessary and without the use of 3-D electroanaotomic mapping systems can be proarrhythmic in many patients. However, in the vast majority of patients with AF necessitating linear ablation after PVI, anatomic targets cannot be accurately defined by fluoroscopy alone due to the limited number of projections and to beat-by-beat variation in catheter contact. In addition, validation of linear lesions without use of 3-D systems may be challenging and time-consuming. In our experience, 3-D electroanatomic systems permit on-line monitoring of the catheter tip with accurate lesion positioning and tracking, which results in rapid identification and ablation of all anatomic targets with shorter procedural and fluoroscopic times and lower RF energy application. Since 1999 we have routinely performed linear ablation guided by 3-D electroanatomic systems, which ensure continuity and transmurality of lesions around PVs and anywhere in the LA, which translates to better outcome, as reported in prior studies from our group in a large number of patients with AF.6, 14, 15, 20, 21 Between 1999 to 2011 we performed more than 25 000 AF ablation procedures with higher success rates from a single procedure using linear ablation strategy and our excellent results have been recently confirmed by other groups without use of 3-D mapping systems, but repeat procedures were required to achieve a 90% success rate. We believe that routine use of 3-D electroanatomical mapping systems is desirable in any modern electrophysiology laboratory to perform linear lesions that minimize the amount of ablation and make the procedure easier and less “blind” (Figure 1).
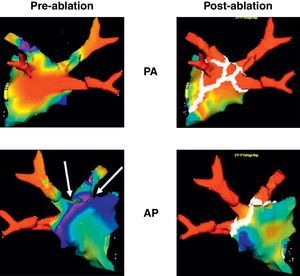
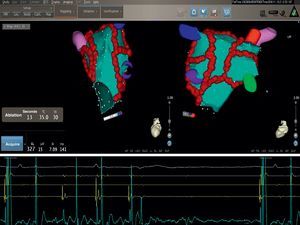
Figure 1. Electroanatomic 3-dimensional (NavX®) reconstruction of the left atrium before (left) and after (right) catheter ablation in 2 projections showing the standard set of lesions (white dots), as performed in circumferential pulmonary vein ablation. Seen clearly are the tubular portions of each of the 4 pulmonary veins in all projections. Note that some areas as seen from the 3-dimensional posterior aspect show low voltage (<0.1mV, red areas) before ablation, indicating multiple areas of fibrosis. AP, anteroposterior view; PA, postero-anterior view.
Catheter ablation strategy in paroxysmal atrial fibrillationCatheter ablation is now widely practiced as a definitive treatment for paroxysmal AF.1 Usually, the vast majority of patients with paroxysmal AF referred to our laboratory for catheter ablation are symptomatic with frequent recurrences, presence of structural heart diseases, comorbidities, and enlarged atria; only a minority has lone AF. In such a patient population we perform CPVA which consists of PVI and additional linear ablation by an irrigated tip catheter guided by 3-D electroanatomic systems to further reduce substrate for reentry (Figure 1, Figure 2, Figure 3). Isolation of PVs represents the first step and circumferential linear lesions are performed point-by-point to encircle the left and right PVs individually or as ipsilteral pairs in accordance with the venous anatomy and operator's preference in order to electrically disconnect all PVs (Figure 1, Figure 2, Figure 3). Complete distal electrical isolation can be achieved by atrial potential abatement (>90% reduction of electrogram amplitude) and electrogram amplitude decrease of <0.1mV around and within the encircled areas (Figure 2, Figure 3), as validated by Lasso catheter.29 After PVI we perform additional lesions, such as one connecting the two circular lesions that surround the pulmonary veins and one connecting the mitral valve annulus to the circular lesion that surrounds the left PV. Point-by-point lesions along the back and the roof of LA between the two sets of PVs connecting the superior and inferior PVs and the mitral valve annulus. The mitral isthmus line is deployed by creating a linear ablation line joining the lateral mitral annulus to the left inferior PV (Figure 1) to further reduce the substrate as well as to prevent postablation macroreentrant left AT.28 Completeness of the mitral isthmus line (bidirectional mitral isthmus conduction block) is a crucial end point and should be validated during coronary sinus (CS) pacing by endocardial and CS mapping, looking for widely spaced double potentials across the line of block, and confirmed by differential pacing.28 Ablation of the mitral isthmus and validation of the block may be challenging, but with the routine use of 3-D systems the catheter stability is ensured, only rarely requiring ablation within the CS to achieve mitral isthmus block. During the procedure all vagal reflexes are eliminated, which enhances the efficacy of the procedure in the long-term outcome (Figure 4). Non-PV triggers are identified in a minority of patients. If AF persists after PVI and additional standard lesions and/or potential non-PV triggers identification, electrical cardioversion is performed and is successful in all patients.
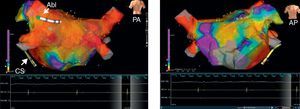
Figure 2. The figure shows detailed 3-dimensional electroanatomic bipolar voltage maps using the NavX® fusion system in 2 projections after standard circumferential pulmonary vein ablation. A significant voltage abatement is evident on targeted areas as peformed in circumferential pulmonary vein ablation. No potential abatement is found on the anterior wall (right panel). Voltages are color coded according to corresponding color bars. In the color scale, red represents areas of lowest voltage potentials (bipolar cutoff value of <0.1mV) while violet represents areas of highest potentials. Abl, ablation; AP, anteroposterior view; CS, coronary sinus; PA, postero-anterior view.
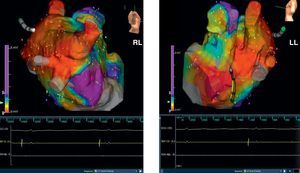
Figure 3. Same patient as in Figure 2 . Three-dimensional electroanatomic voltage maps after circumferential pulmonary vein ablation in the right lateral and latero-lateral projections are shown. LL, latero-lateral; RL, right lateral.
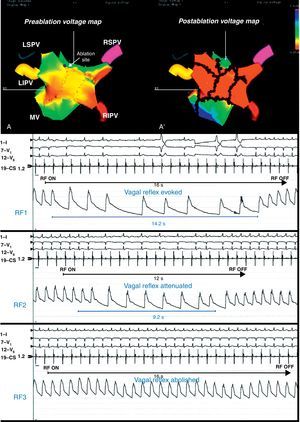
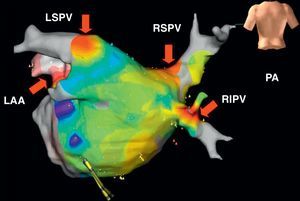
Figure 4. Preablation (A) and postablation (A’) 3-dimensional electroanatomic voltage maps in a patient with vagal reflex evoked and abolished around the left superior pulmonary vein (arrow). Reproduced with permission from Pappone et al. 22 LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MV, mitral valve; RF, radiofrequency; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Catheter ablation strategy in persistent atrial fibrillationAlthough we suggest catheter ablation as a first-line therapy in selected patients with AF, we do not believe that this treatment strategy should be offered first to all patients with AF as the potential benefit is highly operator dependent, particularly in patients with persistent AF. Since PVI in persistent arrhythmia may be associated with high recurrence rates, in the last few years new hypothesis-based ablation strategies including additional linear ablation after PVI have been proposed to improve the long-term outcome of this patient population. The usefulness of additional linear lesions in patients with chronic AF was first demonstrated by our group in a randomized multicenter study.7 In the stepwise approach, which includes multiple sequential linear lesions after PVI, critical areas are sequentially identified by the impact of ablation on AF cycle length (CL) prolongation so that the atrium can no longer sustain the fibrillatory process and the arrhythmia terminates either by direct conversion to sinus rhythm or, more frequently, to an AT which can be mapped and definitively ablated. Validation of lines by bidirectional block may be challenging, but it is necessary because incomplete block facilitates the development of postablation ATs. The end point of the procedure is reversion to sinus rhythm or to an intermediate AT; if the end point is not achieved after the first linear lesion, additional lesions are performed. Currently, the stepwise strategy has become the standard approach for patients with persistent longstanding AF in many laboratories including our own.
Epicardial Coronary Sinus AblationAblation inside the CS (epicardial ablation) is performed if CS potentials persist. Usually RF applications start distally (4 o’clock in left anterior oblique view) up to the ostium by targeting local, rapid, sharp and fractionated potentials (Figure 5). Additional RF applications are deployed around the CS ostium from the right atrium. Power is limited to a maximum of 25W and the irrigation rate is adjusted manually to achieve the desired power setting. Coronary sinus disconnection is validated by the dissociation or abolition of sharp potentials in the first 3 cm.
Figure 5. Electroanatomical maps using the CartoMerge® system in 2 projections (latero-lateral in the left and postero-anterior view in the right) in a patient with persistent atrial fibrillation. At the end of the standard set of lesions (red dot lines), as performed by circumferential pulmonary vein ablation, atrial firbillation converts to an intermediate atrial tachycardia. Immediately after starting radiofrequency application at the coronary sinus ostium (ablation catheter tip in red) atrial tachycardia immediately terminates with sinus rhythm restoration.
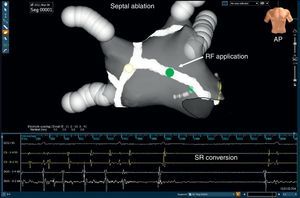
Septum ablationLinear ablation of the interatrial septum is performed at operator discretion at sites of continuous electrical activity, complex fractionated activity, and areas with CL shorter than the mean left atrial appendage (LAA) CL. The RF application starts from the anterior aspect of the lesion encircling the right superior PV up to the anterior mitral annulus (Figure 6). Like CS ablation, the end point is elimination of local electrograms or alternatively to prolong the CL in this region; validation of complete conduction block across this lesion is not routinely performed. During ablation of the anterior septum, RF applications close to the His bundle should be avoided.
Figure 6. An electroanatomical map using the NavX® system (gray) in anteroposterior tilting view in a patient with persistent atrial fibrillation shows a septal ablation line (white) which after radiofrequency application at the medium septum (green dot) reverted to sinus rhythm by an intermediate atrial tachycardia. AP, anteroposterior view; RF, radiofrequency; SR, sinus rhythm.
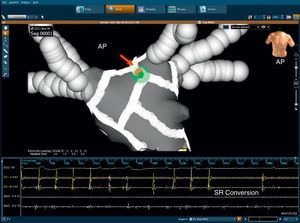
Left atrial ablationAblation is typically performed beginning from the lesion encircling the left superior PV and then the lesion is extended inferiorly and superiorly. The end point is complete elimination of local endocardial electrograms bordering the LAA—posterior, inferior, and anterior—to increase the CL of sharp potentials characteristically found within the LAA (Figure 7).
Figure 7. An electroanatomical map using the NavX Dyne CT® system (gray) in a patient with persistent atrial fibrillation (anteroposterior projection). Note that at the end of left atrial appendage the encircling atrial fibrillation/atrial tachycardia reverted to sinus rhythm. AP, antero-posterior view; RF, radiofrequency; SR, sinus rhythm.
Endocardial coronary sinus ablationLinear ablation is performed beginning along the endocardial aspect of the CS and is completed from within the vessel. The ablation catheter is dragged along the endocardium of the inferior LA parallel to the CS catheter. The RF application begins at the inferior LA along the posterior mitral annulus, proceeding near to the CS ostium up to the lateral LA (4 o’clock in the left anterior oblique projection). The end point is complete elimination of sharp fractionated and rapid potentials inside the CS.
Linear right atrium ablationIn a minority of patients (<10%) atrium areas outside the LA such as right atrium or superior vena cava may be responsible for potential perpetuators of AF. Differences in CL between right atrium appendage and LAA (CL measured in the right atrium shorter [>20ms] than that measured in the LA) may suggest that right atrium should be mapped and ablated. Linear lesion may be deployed between the inferior vena cava and the tricuspid annulus isthmus to create a bidiretional conduction block across the isthmus. Validation of bidirectional block is performed during sinus rhythm. Superior vena cava isolation is not routinely performed, but only in the presence of arrhythmogenic sources.
Atrial fibrillation cycle length monitoringThe effect of RF applications is continuously monitored by assessing changes of AF CL before and after each ablation step by averaging 10 consecutive cycles and at the time of AF termination. The AF CL is determined from within the CS and the right and left atrial appendages. A single site is considered to have a significant impact if its ablation results in termination of AF or prolongation of the AF CL (evaluated in the LAA except if specified otherwise) by 20 ms or more, when compared to the highest AF CL during the previous steps. Although a specific sequence is not crucial, in our experience the CS, the interatrial septum, and the base of the LAA are the key areas to terminate AF, all of which should be included in any linear ablation strategies for persistent AF (Figure 6, Figure 7).
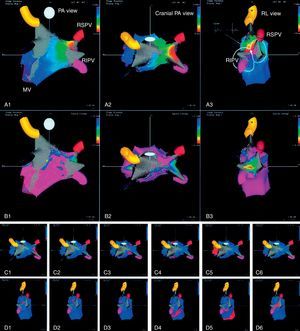
Postablation new-onset atrial tachycardiasIn paroxysmal AF, PVI, and/or CPVA may result in the development of postablation new-onset ATs in a minority of cases, mostly depending on the presence of residual gaps on the lesion lines. In our experience with CPVA, postablation ATs are transient, but in about 5% of cases are incessant and poorly tolerated requiring a redo procedure with an activation map to eliminate them. Conventional or 3-D electroanatomical activation maps have demonstrated that many postablation ATs in patients with paroxysmal AF are macroreentrant (Figure 8), but in some patients a focal or multifocal mechanism is found (Figure 9). In patients with persistent AF, sequential linear lesions may facilitate termination of AF but also transition to one or more ATs, which does not necessarily represents a proarrhythmic phenomenon but is a necessary step before sinus rhythm restoration. We routinely use 3-D mapping systems in the treatment of patients with persistent AF permitting online analysis and color mapping of ATs. If a focal AT is the arrhythmia being mapped, the earliest activation time is the site of ablation. At sites where ablation of focal AT is successful, usually the earliest bipolar electrogram in the red region precedes the P wave by at least 30ms. However, macroreentrant ATs require accurate mapping and cautious interpretation of color-coded activation maps. If the entire circuit of macroreentrant ATs is completely mapped, the red area (earliest activation) should be adjacent to a purple area (latest activation) to complete the circuit. In our laboratory, we usually take at least 150 points for macroreentrant ATs and less than 100 points for the focal ones and a stable intracardiac atrial electrogram is generally chosen. The AT transition mainly depends on the amount of ablated area, and its occurrence is more frequent in patients undergoing a more extensive ablation. At the time of AT transition, conventional activation mapping with entrainment pacing is usually performed to include or exclude macroreentry as the arrhythmia mechanism. In our experience, during stepwise extensive ablation a gradual prolongation of AF CL usually occurs with the largest increments observed during RF applications on the interatrial septum, CS region, and LAA region, in many cases resulting in conversion to SR without intermediate AT. Postablation ATs are usually macroreentrant perimitral or roofline28, 30 and rarely the cavotricuspid isthmus is involved; focal ATs are less frequently found and in many cases are located at the PVs or LAA.28, 30 In many experienced centers, a stepwise ablation strategy is a useful approach for the treatment of patients with long-standing persistent AF, enlarged atria, and comorbidities. However, repeat procedures are usually required to achieve high success rates. Therefore, early catheter ablation including PVI and less extensive linear ablation as performed in CPVA can be considered as a new indication in patients with AF at risk to rapidly progress to persistent AF.
Figure 8. Activation (A1–A3), voltage (B1–B3), and propagation (C1–C6, D1–D6) maps of macroreentrant left atrial tachycardia with critical isthmus between right superior and right inferior pulmonary veins. Reproduced with permission from Pappone et al. 28 . MV, mitral valve; PA, postero-anterior view; RIPV, right inferior pulmonary vein; RL, right lateral view; RSPV, right superior pulmonary vein.
Figure 9. An electroanatomical activation map using the NavX Dyne CT® system in postero-anterior projection in a patient with persistent atrial fibrillation showing multifocal atrial tachycardias from pulmonary veins and left atrial appendage (red arrows). In the color scale, regions of red color indicate sites of earliest activation and successful ablation. LAA, left atrial appendage; LSPV, left superior pulmonary vein; PA, postero-anterior view; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Future directionsBecause of the difficulties with long-term antiarrhythmic and anticoagulation drug therapy, we believe that catheter ablation will evolve to a front-line therapy in many patients with AF. At present, high success rates have been achieved by a single procedure in many centers worldwide in paroxysmal AF with both manual and remote mapping and ablation,31, 32, 33, 34 but in persistent long-standing AF the catheter ablation strategy is more complex and time consuming, frequently requiring repeat procedures to achieve a success rate as high as in paroxysmal AF. In the near future, however, with growing experience and evolving technology, catheter ablation of AF also may be extended to patients with long-standing AF; however, we believe that identification and evaluation of the amount of LA scarring or fibrosis is crucial to avoid unnecessary procedures in this patient population.
Conflicts of interestProf. Carlo Pappone has relationship with Biotronik and St.Jude Medical. Dr. Vincenzo Santinelli has no conflict of interest to declare.
Received 15 December 2011
Accepted 17 December 2011
Corresponding author: Dipartimento di Aritmologia, Maria Cecilia Hospital, Via Corriera 1, 48010 Cotignola, Ravenna, Italy. cpappone@gvm-vmc.it