To determine the dose-response association between current and past leisure-time physical activity (LTPA), total and at different intensities, and high-density lipoprotein (HDL) functionality parameters.
MethodsStudy participants (n=642) were randomly drawn from a large population-based survey. Mean age of the participants was 63.2 years and 51.1% were women. The analysis included data from a baseline and a follow-up visit (median follow-up, 4 years). LTPA was assessed using validated questionnaires at both visits. Two main HDL functions were assessed: cholesterol efflux capacity and HDL antioxidant capacity, at the follow-up visit. Linear regression and linear additive models were used to assess the linear and nonlinear association between LTPA and HDL functionality.
ResultsTotal LTPA at follow-up showed an inverse and linear relationship between 0 and 400 METs x min/d with HDL antioxidant capacity (regression coefficient [beta]: −0.022; 95%CI, −0.030, −0.013), with a plateau above this threshold. The results were similar for moderate (beta: −0.028; 95%CI, −0.049, −0.007) and vigorous (beta: −0.025; 95%CI, −0.043, −0.007), but not for light-intensity LTPA. LTPA at follow-up was not associated with cholesterol efflux capacity. Baseline LTPA was not associated with any of the HDL functionality parameters analyzed.
ConclusionsCurrent moderate and vigorous LTPA showed a nonlinear association with higher HDL antioxidant capacity. Maximal benefit was observed with low-intermediate doses of total LTPA (up to 400 METs x min/d). Our results agree with current recommendations for moderate-vigorous LTPA practice and suggest an association between PA and HDL functionality in the general population.
Keywords
Regular leisure-time physical activity (LTPA) is related to a lower risk of cardiovascular disease and all-cause mortality.1–4 Physical inactivity causes an estimated 6% of the burden of coronary heart disease and 9% of the premature mortality burden.5 The World Health Organization recommends that all adults undertake 150-300minutes of moderate-intensity or 75-150minutes of vigorous-intensity physical activity (PA), or some equivalent combination of moderate-intensity and vigorous-intensity aerobic PA, per week.6 Moreover, PA should be incorporated and performed regularly across the lifespan.6
PA improves cardiometabolic clinical phenotypes such as lipid profile, blood pressure, carbohydrate metabolism, hemostasis, and inflammation.7 However, the mechanisms through which PA induces cardiovascular health benefits are still not fully understood.7,8 One of the best-known effects of PA is to increase levels of high-density lipoprotein cholesterol (HDL-C).9 HDL-C levels have been consistently and inversely related to cardiovascular risk in observational studies, but Mendelian randomization and experimental studies have questioned the causality of this association.10,11 Therefore, the antiatherogenic properties of HDL particles could be related to the qualitative and functional characteristics of the lipoprotein rather than the quantity of HDL-C.12 Among these functional characteristics, cholesterol efflux capacity (CEC)13 and HDL antioxidant capacity (HAC)14,15 have been related to cardiovascular risk.
The relationship between LTPA and HDL functionality parameters has been analyzed in several studies.16–20 However, the dose-response pattern of the association considering current and past LTPA, and PA intensities in a population-based study has not been previously addressed. The aim of this study was to determine the dose-response association between current and past LTPA and HDL functionality in a population-based sample. We also analyzed the importance of PA intensity (light, moderate or vigorous) in this association.
METHODSStudy design and populationThe Registre Gironí del Cor (REGICOR) study, begun in 1978, aims to enhance understanding of the epidemiology of cardiovascular disease.21 One of the components of the REGICOR study is a population-based cohort including 6352 individuals recruited between 2003 and 2006 and reexamined between 2008 and 2013 (4280 attended). Participants were aged 35 to 79 years and were resident in the referral area.
In this analysis, we included a random subsample of 642 individuals who participated in both exams. In this subset of participants, HDL functionality traits were measured. The study was approved by the local ethics committee and all participants provided their written informed consent.
Measurement of leisure-time physical activityThe Minnesota Leisure-Time Physical Activity Questionnaire was used to measure PA practice at the baseline visit. This questionnaire has been validated for the Spanish adult population22,23 and assesses leisure and active commuting domains and frequency, duration, and intensity dimensions. Briefly, from a list of 64 activities, participants marked those they had practiced during the year prior to the visit, and a trained interviewer collected information related to the frequency of practice and the duration of each session. Each PA is assigned an intensity based on metabolic equivalents of task (MET).24 The Minnesota Leisure-Time Physical Activity Questionnaire allows estimation of the the daily average energy expenditure in the previous year (METs x min/d) and further classification as light-intensity LTPA if the activity requires <4 METs (eg, slow-paced walking), moderate-intensity LTPA if it requires 4 to 5.9 METs (eg, brisk walking), and vigorous-intensity LTPA if it requires ≥ 6 METs (eg, jogging).25 Thus, for each participant we estimated:
Total LTPA=light-intensity LTPA + moderate-intensity LTPA + vigorous-intensity LTPA
At the follow-up visit, a short version of the Minnesota Leisure-Time Physical Activity Questionnaire was administered. The short version collects data about the monthly frequency, and average daily duration of practice of 6 types of PA: walking, brisk walking, gardening, walking trails, climbing stairs, and sports activities. This short version provides the same information as the original questionnaire and has been validated in the Spanish population.26 In the validation study, the Spearman correlation coefficients between the extended and the short questionnaires were 0.82 for total LTPA, 0.89 for light LTPA, 0.79 for moderate LTPA, and 0.68 for vigorous LTPA. The short questionnaire also includes 2 questions on sedentary behavior and 1 on occupational PA that were not considered in this analysis.
HDL functionality traitsWe measured CEC and HAC in apolipoprotein-B depleted plasma at the 2008 to 2013 follow-up visit as previously described.27
Preparation of apolipoprotein-B depleted plasmaAll HDL functionality experiments were performed in apolipoprotein-B depleted plasma (ABDP), which contains only high-density lipoproteins. Plasma from the participants was incubated with a suspension of 20% polyethylene glycol 8000 (Sigma, United States) in a 200mM glycine buffer pH 7.4 (Sigma), at 4°C for 20minutes. The mixture was then centrifuged (10 000rpm, 15minutes, 4°C); supernatants were collected and finally stored at −80°C upon use.
Cholesterol efflux capacityTHP-1 monocytes were grown in RPMI 1640 medium, supplemented with 10% heat inactivated FBS, 1% sodium pyruvate, 1% L-glutamine, and 1% penicillin-streptomycin. Cells were refreshed every 72hours. Monocytes were differentiated into macrophages through their incubation with phorbol-myristate-acetate (Sigma) 200nM for 96hours. THP-1 monocyte-derived macrophages were then incubated with 0.2μCi/mL of [1,2-3 H(N)]-cholesterol (Perkin-Elmer, United States) for 24hours, washed, incubated in fresh RPMI 1640 medium supplemented with 1% bovine serum albumin (BSA, Sigma) for 24hours, washed again, and finally cultured in fresh RPMI 1640 medium + 1% BSA in the presence of 5% ABDP from the participants, or without (control), for 16hours. The culture supernatants were obtained, and the cell culture lipids were extracted with ice-cold isopropanol for 60minutes. Radioactivity in both supernatant medium and cell lipids was measured in a beta scintillation Tri-Card 2800TR counter (Perkin-Elmer). Finally, CEC for each well was calculated according to this equation:
We ran samples in duplicate and the mean value was considered for the analyses. We also corrected interassay variation by a pooled ABDP normalization as follows: a pool of ABDP obtained from 20 healthy volunteers was included in each experiment as interassay control, and we divided the values of all cholesterol efflux results of the volunteers by the efflux value of this pool. The interassay coefficient of variation of this pooled normalization method was 9.6%.
HDL antioxidant capacity measurementHDL antioxidant capacity (HAC) was measured following the “HDL inflammatory index” technique. In brief, we measured the capacity of participants’ HDL to avoid the oxidative modification of 2’-7’-dichlorodihydrofluorescein diacetate (H2DCF-DA, Life Technologies, Thermo Fisher Scientific, United States) in the presence of oxidized low-density lipoproteins (LDL). H2DCF-DA was diluted in methanol (final concentration: 2mg/mL) for 30minutes, to obtain its deacetylated form (H2DCF). Oxidized LDL was prepared from a pool from plasma from 20 healthy participants by density gradient ultracentrifugation. LDL were oxidized, diluted to 100mg/L and stored at −80°C upon use. Finally, 5μL of ABDP from the volunteers was incubated with H2DCF (final concentration: 3μg/mL) and oxidized LDL (final concentration: 1.5μg/mL) in 96-well, black polystyrene plates, at 37°C. Fluorescence was measured every 3minutes for 75minutes in an Infinite M200 reader (Tecan Ltd, Switzerland). The greater the oxidation of H2DCF, the greater the fluorescent signal and the lower the HDL antioxidant capacity. To calculate the antioxidant capacity of HDL, the slope between 15 and 75minutes was calculated (the relationship between fluorescence and time was linear between these times). We analyzed samples in duplicate and the mean value was used for the analyses. We also corrected interassay variation by a pooled ABDP normalization. The interassay coefficient of variation of this pooled normalization method was 4.6%.
CovariatesTrained personnel administered a series of validated questionnaires and carried out measurements following a standardized method to collect information on sociodemographic (age, residence, sex, educational level), lifestyle (smoking status, diet) and anthropometric variables (weight, height, body mass index), blood pressure, and drug treatments. In addition, a series of complementary laboratory tests on serum were carried out, including total cholesterol, HDL-C, LDL cholesterol, triglycerides, and glucose. These assessments were performed at both visits (figure 1).
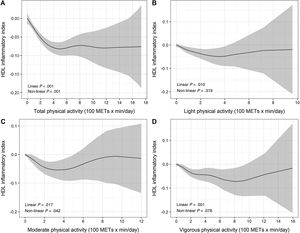
Dose-response association of different intensities of current leisure-time physical activity (total, and light-, moderate- and vigorous-intensity—100 METs x min/d—) and HDL antioxidant capacity, assessed by restricted cubic splines. The black line represents the estimated effect size of the association and the grey area the 95% confidence interval. HDL, high-density lipoproteins; HDL, high-density lipoprotein; MET, metabolic equivalent of task.
Quantitative variables are presented as mean and standard deviation or median and interquartile range, while categorical variables are presented as counts and percentages. In the bivariate analyses, Spearman correlation was used to determine the association between 2 quantitative variables and to analyze the linear trend of the association between LTPA quartiles and the variables of interest and covariates, and chi-square tests to compare proportions between groups. To assess the dose-response pattern of the association of PA with HDL functionality, linear regression and additive regression models were fitted. Additive regression models allow exploration of nonlinear relations between an independent continuous trait and a dependent outcome (binary or continuous) based on a number of knots, points in which the association deviates from linearity. We defined a maximum of 3 knots to avoid overfitting and enhance the interpretability of the model. The nonlinear dose-response pattern of the association was assessed visually and also considering the P value. When the pattern of the association was nonlinear, the analysis was split using the visual value that best defined the knot, and 2 conventional linear regression models were used: one to explore the association when LTPA was in the range of values from 0 METs x min/d to the knot, and another model to explore the association when LTPA was in the range of values from the knot to higher values.
The dependent variables were those of HDL functionality: CEC, HAC. The independent variables of interest were, on the one hand, past LTPA, and, on the other hand, current LTPA. Moreover, physical activity was considered as total LTPA, independently of its intensity, in one model, and considering the intensity of PA in the other model, which included LTPA in light-intensity PA, in moderate-intensity PA, and in vigorous-intensity PA. Classic cardiovascular risk factors (age, sex, smoking, diabetes, LDL cholesterol) as well as HDL-C were included as covariates in the multivariable models. Moreover, we designed 2 models, differentiated by the exclusion (model 1) or inclusion (model 2) of body mass index to explore the potential mediating effect of this variable.
R software (Version 4.0.3) and Rstudio were used for the statistical analyses. For the linear component, a P value <.008 was considered statistically significant after considering multiple comparisons (3 independent PA variables–light, moderate and vigorous LTPA– and 2 independent parameters of HDL functionality=6; 0.05/6=0.008).
RESULTSStudy populationThe characteristics of the 642 participants at the follow-up visit across total LTPA quartile groups are shown in table 1. The proportion of men and the HDL-C concentration increased across the quartiles of total PA practice, whereas body mass index and HAC decreased as LTPA increased. CEC was similar across total LTPA quartiles.
Characteristics of the participants at the follow-up visit across total leisure time physical activity practice quartiles (METs x min/day)
| Total leisure-time physical activity (METs x min/day) | |||||||
|---|---|---|---|---|---|---|---|
| All (n=642) | Q1 (n=161) | Q2 (n=160) | Q3 (n=161) | Q4 (n=160) | P | N | |
| (0-44.56) | (44.56-154.51) | (154.51-359.64) | (359.64-1733.27) | ||||
| Age | 63.2±11.7 | 62.6±12.3 | 62.8±12.5) | 62.5±11.9 | 64.9±10.1 | .114 | 642 |
| Sex | <.001 | 642 | |||||
| Male | 314 (48.9) | 58 (36.0) | 76 (47.5) | 82 (50.9) | 98 (61.3) | ||
| Female | 328 (51.1) | 103 (64.0) | 84 (52.5) | 79 (49.1) | 62 (38.8) | ||
| Smoking status | .682 | 642 | |||||
| Never | 340 (53.0) | 83 (51.6) | 81 (50.6) | 92 (57.1) | 84 (52.5) | ||
| Current or exsmoker (< 1 y) | 107 (16.7) | 34 (21.1) | 33 (20.6) | 18 (11.2) | 22 (13.8) | ||
| Exsmoker (> 1 y) | 195 (30.4) | 44 (27.3) | 46 (28.7) | 51 (31.7) | 54 (33.8) | ||
| Diabetes | 86 (13.4) | 25 (15.5) | 24 (15.0) | 22 (13.7) | 15 (9.38) | .100 | 642 |
| BMI, kg/m2 | 26.9±4.05 | 27.7±4.62 | 26.8±4.07 | 26.6±3.55 | 26.5±3.83 | .007 | 640 |
| Total cholesterol, mg/dL | 209±36.4 | 212±37.3 | 206±35.2 | 209±35.5 | 208±37.5 | .524 | 642 |
| HDL cholesterol, mg/dL | 53.0±12.3 | 51.5±12.2 | 52.5±12.4 | 54.3±12.3 | 53.8±12.4 | .045 | 642 |
| LDL cholesterol, mg/dL | 135±32.2 | 140±32.9 | 133±31.5 | 135±32.2 | 135±32.1 | .264 | 638 |
| Triglycerides, mg/dL | 89.0 [67.0-121] | 94.0 [68.0-125] | 89.0 [69.0-121] | 89.0 [66.0-122] | 82.5 [62.5-115] | .039 | 642 |
| Glycaemia, mg/dL | 97.7±20.5 | 97.2±19.4 | 98.8±23.7 | 98.7±22.1 | 96.1±15.8 | .635 | 642 |
| SBP, mmHg | 131±18.5 | 131±20.8 | 128±17.7 | 130±18.0 | 133±17.0 | .183 | 642 |
| DBP, mmHg | 76.0±9.92 | 76.1±11.2 | 75.2±9.83 | 75.5±9.34 | 77.3±9.20 | .281 | 642 |
| LTPA | |||||||
| Total, METs x min/d | 155 [44.6-360] | 17.5 [3.50-30.9] | 94.4 [65.0-120] | 240 [195-288] | 555 [443-714] | <.001 | 642 |
| Light, METs x min/d | 30.4 [0.00-95.9] | 4.00 [0.00-12.0] | 36.0 [6.88-71.9] | 63.9 [16.0-160] | 95.9 [0.00-240] | <.001 | 642 |
| Moderate, METs x min/d | 1.93 [0.00-61.8] | 0.00 [0.00-5.79] | 8.14 [0.00-39.1] | 0.00 [0.00-79.9] | 79.9 [0.00-282] | <.001 | 642 |
| Vigorous, METs x min/d | 21.0 [1.79-149] | 2.31 [0.00-7.96] | 14.0 [1.36-42.2] | 70.1 [6.99-160] | 242 [90.4-429] | <.001 | 642 |
| Cholesterol efflux capacity | 0.92±0.12 | 0.93±0.12 | 0.92±0.11 | 0.92±0.12 | 0.93±0.12 | .765 | 642 |
| HDL antioxidant capacity | 1.08±0.12 | 1.11±0.12 | 1.10±0.12 | 1.06±0.12 | 1.05±0.11 | <.001 | 642 |
Values are expressed as No. (%), mean±standard deviation, median [interquartile range], or (minimum and maximum value of LTPA in each quartile).
BMI, body mass indx; DBP, diastolic blood pressure; HDL cholesterol, high-density lipoprotein cholesterol; LDL cholesterol, low-density lipoprotein cholesterol; LTPA, leisure-time physical activity; SBP, systolic blood pressure.
Table 2 shows the Spearman correlation coefficients of the association between all the variables of interest, including the covariates, at the follow-up visit. CEC was directly associated with total cholesterol and HDL-C concentrations, and inversely associated with glycemia and triglyceride levels. HAC was directly associated with body mass index, blood pressure, glycemia, and triglyceride levels, and inversely associated with HDL-C levels and with total, light, and vigorous LTPA.
Spearman correlation (rho coefficient, above the diagonal; P value, below the diagonal) between variables of interest at the follow-up visit
| LTPA (METs x min/d) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | BMI | SBP | DBP | Glycemia | Total-C | HDL-C | LDL-C | TG | TotalPA | LightPA | Mod.PA | Vig.PA | CEC | HAC |
| 1 | 0.126 | 0.420 | −0.149 | 0.179 | −0.051 | −0.022 | −0.077 | 0.107 | 0.058 | 0.224 | −0.100 | −0.157 | −0.013 | −0.033 |
| 0.001 | 1 | 0.274 | 0.192 | 0.319 | −0.030 | −0.259 | −0.010 | 0.308 | −0.117 | −0.006 | −0.076 | −0.152 | −0.089 | 0.121 |
| 0.000 | 0.000 | 1 | 0.514 | 0.314 | 0.017 | −0.111 | 0.010 | 0.191 | 0.062 | 0.103 | −0.036 | −0.063 | 0.043 | 0.130 |
| 0.000 | 0.000 | 0.000 | 1 | 0.114 | 0.110 | −0.086 | 0.104 | 0.146 | 0.057 | −0.027 | 0.063 | 0.067 | 0.013 | 0.119 |
| 0.000 | 0.000 | 0.000 | 0.004 | 1 | −0.016 | −0.210 | −0.023 | 0.262 | −0.003 | 0.079 | −0.023 | −0.101 | −0.118 | 0.177 |
| 0.195 | 0.446 | 0.672 | 0.005 | 0.687 | 1 | 0.315 | 0.942 | 0.245 | −0.042 | −0.075 | 0.035 | 0.019 | 0.167 | −0.023 |
| 0.578 | 0.000 | 0.005 | 0.029 | 0.000 | 0.000 | 1 | 0.114 | −0.418 | 0.062 | −0.014 | 0.063 | 0.064 | 0.492 | −0.173 |
| 0.053 | 0.792 | 0.808 | 0.008 | 0.569 | 0.000 | 0.004 | 1 | 0.177 | −0.052 | −0.093 | 0.027 | 0.031 | 0.036 | −0.017 |
| 0.007 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1 | −0.096 | 0.034 | −0.054 | −0.108 | −0.138 | 0.182 |
| 0.142 | 0.003 | 0.118 | 0.152 | 0.948 | 0.287 | 0.119 | 0.193 | 0.015 | 1 | 0.448 | 0.366 | 0.635 | −0.007 | −0.192 |
| 0.000 | 0.888 | 0.009 | 0.487 | 0.046 | 0.059 | 0.722 | 0.019 | 0.387 | 0.000 | 1 | −0.113 | 0.059 | −0.065 | −0.089 |
| 0.011 | 0.054 | 0.361 | 0.113 | 0.557 | 0.381 | 0.109 | 0.495 | 0.169 | 0.000 | 0.004 | 1 | 0.129 | 0.009 | −0.015 |
| 0.000 | 0.000 | 0.110 | 0.092 | 0.011 | 0.627 | 0.108 | 0.440 | 0.006 | 0.000 | 0.138 | 0.001 | 1 | 0.022 | −0.090 |
| 0.735 | 0.025 | 0.274 | 0.745 | 0.003 | 0.000 | 0.000 | 0.361 | 0.000 | 0.851 | 0.101 | 0.813 | 0.585 | 1 | −0.048 |
| 0.399 | 0.002 | 0.001 | 0.003 | 0.000 | 0.559 | 0.000 | 0.668 | 0.000 | 0.000 | 0.024 | 0.699 | 0.022 | 0.225 | 1 |
BMI, body mass index; CEC, cholesterol efflux capacity; DBP, diastolic blood pressure; HAC, HDL antioxidant capacity; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Light PA, light physical activity; Mod. PA, moderate physical activity; SBP, systolic blood pressure; TG, triglycerides; Total-C, total cholesterol; Total PA, total physical activity; Vig. PA, vigorous physical activity.
The linear and nonlinear dose-response relationship between LTPA and CEC is shown in figure 1 of the supplementary data. In the multivariable linear regression analyses, there were no significant associations between past or current LTPA and CEC (table 3).
Relationship between past and current physical activity (total and by intensity), and cholesterol efflux capacity and HDL antioxidant capacity, adjusted for confounding variables
| Cholesterol efflux capacity | HDL antioxidant capacity | |||||
|---|---|---|---|---|---|---|
| β | 95%CI | P | β | 95%CI | P | |
| Total physical activity | ||||||
| Past total LTPA (100 METs x min/day) | 0.001 | −0.002-0.004 | .404 | −0.001 | −0.004-0.003 | .691 |
| Current total LTPA (100 METs x min/day) | 0.000 | −0.003-0.003 | .885 | Nonlinear P <.001 | ||
| <400 METs x min/day current total LTPA | --- | --- | - | −0.022 | −0.030 to −0.013 | <.001 |
| ≥ 400 METs x min/day current total LTPA | --- | --- | - | 0.002 | −0.005-0.008 | .632 |
| Physical activity according to intensity | ||||||
| Past physical activity practice | ||||||
| Past light LTPA (100 METs x min/d) | 0.000 | −0.008-0.007 | .909 | −0.008 | −0.016-0.000 | .039 |
| Past moderate LTPA (100 METs x min/d) | 0.001 | −0.005-0.006 | .791 | 0.001 | −0.005-0.007 | .677 |
| Past vigorous LTPA (100 METs x min/d) | 0.003 | −0.003-0.008 | .310 | 0.001 | −0.004-0.007 | .638 |
| Current physical activity practice | ||||||
| Current light LTPA (100 METs x min/d) | −0.005 | −0.013-0.002 | .166 | −0.011 | −0.019 to −0.003 | .010 |
| Current moderate LTPA (100 METs x min/d) | −0.001 | −0.007-0.004 | .630 | Nonlinear P=.042 | ||
| <200 METs x min/d current moderate LTPA | --- | --- | - | −0.028 | −0.049 to −0.007 | .010 |
| ≥ 200 METs x min/d current moderate LTPA | --- | --- | - | 0.007 | −0.005-0.019 | .265 |
| Current vigorous LTPA (100 METs x min/d) | 0.003 | −0.001-0.007 | .174 | Nonlinear P=.076 | ||
| <200 METs x min/d current vigorous LTPA | --- | --- | - | −0.025 | −0.043 to −0.007 | .007 |
| ≥ 200 METs x min/d current vigorous LTPA | --- | --- | - | −0.004 | −0.012-0.005 | .363 |
Adjusted for age, sex, smoking status, diabetes, HDL-cholesterol, and LDL-cholesterol. B, linear regression coefficient; CI, confidence interval; LTPA, leisure time physical activity.
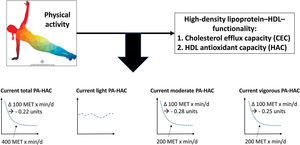
Past LTPA was not associated with HDL antioxidant capacity (HAC) (table 3). The relationship between current total LTPA and the HAC showed both linear and nonlinear components (figure 1A), with a knot (cut-point) around 400 METs x min/d. Below this threshold, total LTPA was inversely associated with HAC: each unit of 100 METs x min/d was associated with a 0.022-unit decrease in HAC; above this threshold, there was no association (table 3).
Consistent with total LTPA results, moderate LTPA and vigorous LTPA showed a nonlinear association with HAC (figure 1C,D), with a knot (cutoff point) around 200 METs x min/d. Below this threshold, moderate and vigorous LTPA showed an inverse and similar magnitude of association with HAC: each 100 METs x min/d was associated with a decrease in HAC of −0.028 and −0.025 units, respectively; above this threshold, there was no association (table 3). Further adjustment by body mass index did not modify the magnitude of the association (table 1 of the supplementary data). Current light LTPA was not associated with HAC (figure 1B and table 3).
DISCUSSIONIn this study, we observed that current total, moderate, and vigorous-intensity LTPA were nonlinearly associated with HAC (figure 2). Current total LTPA shows an inverse and linear relationship between 0 and 400 METs x min/d, with a plateau above this threshold. Similar results were observed for current moderate- and vigorous-intensity LTPA, but not for light-intensity LTPA, with a cutpoint around 200 METs x min/d. Current LTPA was not associated with CEC and past LTPA was not associated with any of the HDL functionality parameters analyzed.
Central illustration. Dose-response association between current total leisure-time physical activity and HDL antioxidant capacity. The dose-response association between light-, moderate- and vigorous-intensity physical activity and HDL antioxidant capacity is also shown. HAC, HDL antioxidant capacity; MET, metabolic equivalent of task; PA, physical activity.
CEC is the capacity of HDL-C to promote reverse cholesterol transport from peripheral cells to the liver.28 Experimentally, CEC quantifies the movement of labeled cholesterol from the inside of the cell to the extracellular medium.17 The effect of LTPA on this process has been previously assessed, with heterogeneous results.17 Our results indicate a lack of association between LTPA and CEC at any intensity level. Hernáez et al.16 reached similar conclusions after analyzing 296 individuals at high cardiovascular risk. However, Khan et al.18 studied the effect of weight loss and exercise in metabolic syndrome patients and concluded that CEC improves after these interventions. Consistent with this finding, other groups have pointed to a beneficial effect of PA on CEC19,29–32 but with some differences in the level of the CEC increase achieved. Most of these studies indicate that moderate- and vigorous-intensity LTPA has the strongest effect on CEC.4,19,32 These inconsistencies could be related to the lack of a standardized method to measure CEC in humans. Most of the studies used murine J774 macrophages for laboratory tests, while the present study and Hernáez et al. used human THP1 monocytes. This heterogeneity could also be partially explained by the design of the studies (experimental vs observational), the type of PA intervention or the method used to measure PA practice, the characteristics of the population included in the studies, the use of concomitant drugs, and dietary differences.
The HAC measures the ability of HDL to prevent LDL oxidation. Therefore, an elevated antioxidant capacity of HDL reduces the oxidation of LDL. HDL antioxidant capacity is inversely associated with cardiovascular death, ischemic heart disease, and hospitalization for myocardial infarction, among others.15 The dose-response effect of LTPA on HAC had not been previously studied in the general population. In our data, we observed that LTPA was associated with decreased HAC values up to 400 METs x min/d. This pattern concurs with the association of LTPA with cardiovascular events and all-cause mortality in the same population4: increasing levels of total LTPA were inversely related to the incidence of cardiovascular events and all-cause mortality until a cutpoint of 400 METs x min/d, beyond which there were no further benefits. Two studies have also reported a shift from pro-oxidant/inflammatory to antioxidant/inflammatory in the HDL profile after a short training program in metabolic syndrome patients (10-week intervention)33 and overweight individuals (3-week intervention).34 Changes observed in the HDL lipidome, proteome, or its structure could mediate the anti-inflammatory and antioxidant capacities of the lipoprotein and consequently modulate cardiovascular risk.18 With respect to the effects of PA intensity, moderate and vigorous LTPA, also related to lower cardiovascular risk in the same REGICOR cohort,4 had a similar magnitude of association with HAC. The association of moderate LTPA with HAC was not statistically significant, likely as a consequence of the low amount and low variability of the practice of this type of PA, hampering the statistical power of our analysis.
Our study has several strengths. Our analysis included a population-based sample, assessed the dose-response pattern of the association between LTPA and HDL functionality parameters, and included different types of LTPA according to their intensity at baseline and at 4 years of follow-up.
The study also has some limitations. This was an observational study and PA was assessed using questionnaires. Although they were validated, some misclassification of the exposure of interest cannot be excluded. Second, the causal relationship between PA and HDL functionality could be difficult to ascertain using an observational approach. The dose-response association, temporal trend (current but not past LTPA), plausibility, and consistency with experimental studies support the causal relationship between PA and HDL functionality; however, we cannot exclude the presence of residual confounding in the estimated effect of this association. Third, HDL functionality was assessed with in vitro techniques, and was limited to cholesterol efflux capacity and HDL antioxidant capacity measured with the HII method, and no other functionality parameters or methods were used (eg, Apo A1, paraoxonase). Finally, the distribution and low variability of moderate-intensity LTPA practice in our sample limits the statistical power of our study.
CONCLUSIONSThis population-based study evaluated the dose-response relationship between LTPA and HDL functionality parameters. Current moderate- and vigorous-intensity LTPA showed a nonlinear association with higher HDL antioxidant capacity (figure 2). Maximal benefit was observed with low-intermediate doses of PA, with a plateau above 400 METs x min/d for total LTPA. Our results agree with current recommendations of low-intermediate doses of moderate-vigorous intensity LTPA practice and suggest an association between PA and HDL functionality in the general population.
FUNDINGThis work was supported by the Carlos III Health Institute–European Regional Development Fund [CIBERCV, CIBEROBN, CIBERESP]; PERIS from Agència de Gestió d’Ajuts Universitaris i de Recerca [SLT002/16/00088]; and the Government of Catalonia through the Agency for the Management of University and Research Grants [2017SGR946].
AUTHORS’ CONTRIBUTIONSContributed substantially to the study conception: R. Viadas, R. Elosua, C. Lassale, Á. Hernáez, S. Sayols-Baixeras. Contributed substantially to the study design: J. Marrugat, H. Schroeder, R. Elosua. Contributed substantially to data acquisition: Á. Hernáez, S. Sayols-Baixeras, J. Marrugat, R. Elosua. Contributed substantially to data analysis: R. Viadas, A. Toloba, R. Elosua. Contributed substantially to the interpretation of the results: R. Viadas, A. Toloba, I. Fernández, S. Sayols-Baixeras, Á. Hernáez, H. Schroeder, I.R. Dégano, C. Lassale, J. Marrugat, R. Elosua. Wrote the article: R. Viadas, R. Elosua. Critical review of the intellectual content: A. Toloba, I. Fernández, S. Sayols-Baixeras, Á. Hernáez, H. Schroeder, I. R. Dégano, C. Lassale, J. Marrugat. Gave final approval of the version to be published: R. Viadas, A. Toloba, I. Fernández, S. Sayols-Baixeras, Á. Hernáez, H. Schroeder, I. R. Dégano, C. Lassale, J. Marrugat, R. Elosua. Agreed to accept responsibility for all aspects of the article and investigate and resolve any issues related to the accuracy and veracity of any part of the work: R. Viadas, A. Toloba, I. Fernández, S. Sayols-Baixeras, Á. Hernáez, H. Schroeder, I. R. Dégano, C. Lassale, J. Marrugat, R. Elosua.
CONFLICTS OF INTERESTThe authors declare they have no conflicts of interest, and that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
- •
Physical activity reduces the risk of coronary artery disease.
- •
Physical activity improves lipid profile and increases HDL-C.
- •
HDL-C levels are not causally related to the risk of coronary artery disease.
- •
The mechanisms explaining the benefits of physical activity are not fully understood.
- •
Current physical activity between 0 and 400 METs x min/d is linearly related to HDL antioxidant capacity with a plateau above this threshold.
- •
Current moderate- and vigorous-intensity physical activity showed a similar pattern of association, whereas light-intensity physical activity was not associated with HDL antioxidant capacity.
- •
Current physical activity was not associated with cholesterol efflux capacity.
- •
Past physical activity was not associated with any of the HDL functionality parameters analyzed.
We thank Elaine M. Lilly, PhD, for her critical reading and revision of the English text.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.04.009