Keywords
INTRODUCTION
It is now generally accepted that inflammation plays a fundamental role in the development and progression of atherosclerotic lesions, and that it is involved in both the short and long term appearance of clinical symptoms.1
Acute heart failure is the main cause of morbidity/mortality in patients with acute myocardial infarction (AMI).2 The latter sets a number of compensatory hormonal and peptide mechanisms in motion that have effects on the kidney, the peripheral vascular system and the myocardium itself.3 In addition, an immune reaction is activated (with systemic, vascular and myocardial components) involving the release of cytokines, mediators of inflammation and growth factors. These phenomena contribute towards the perpetuation of ventricular dysfunction; they must therefore be taken into account when trying to arrive at a prognsosis.4,5
Interleukin 10 (IL-10) is a cytokine with anti-inflammatory properties that inhibits the synthesis of proinflammatory cytokines by T lymphocytes and macrophages, as well as interfering with the other inflammatory functions of these cells. Interleukin 10 has been detected in human atherosclerotic plaques, and in animal experiments low concentrations of this cytokine have been found to favor the development of larger and morphologically less stable atherosclerotic lesions.6 It is also known that IL-10 is released into the bloodstream during post-ischemic myocardial reperfusion and during cardiopulmonary bypass in human patients.7-10 Our group recently demonstrated an association between post-ischemic reperfusion and plasma IL-10 concentrations in patients with AMI treated by primary percutaneous transluminal coronary angioplasty (PTCA).11,12
The present paper examines the relationship between serum IL-10 concentrations in patients with AMI who received successful primary PTCA (i.e., in whom revascularization of the artery involved in the infarction was achieved and a "thrombolysis in myocardial infarction" [TIMI] flow score of 3 was established) and the later development of heart failure.
PATIENTS AND METHODS
Patients
Between May 2002 and May 2003, 89 patients received primary PTCA for AMI at our center. All patients presented with angina-like chest pain of >30 min duration and with an elevation of the ST segment of ≥0.1 mV as registered by 2 or more contiguous leads. Primary PTCA was performed on 65 patients, who made up the study population. The procedure was successful in all cases i.e., a TIMI score of 3 was achieved in the affected artery and the residual stenosis was <30%). All patients gave their consent to be included in the study, which was approved by the ethics committee of our institution.
Patients with concomitant disease, such as infections, acute or chronic kidney or liver disease, cancer or autoimmune disease were excluded. Also excluded were patients diagnosed with cardiogenic shock at admission, and those who had suffered a previous AMI.
Coronary angiography and PTCA were performed using standard techniques. Blood flow in the affected artery was defined using the criteria of the TIMI study.13 Percutaneous transluminal coronary angioplasty was performed when initial angiography of the affected artery showed total occlusion or subtotal occlusion and the TIMI score was <3.
Clinical Variables
The following variables were recorded for all subjects: a) demographic characteristics--age and sex--; b) coronary risk factors--high blood pressure, dyslipidemia, diabetes, and smoking habit--; c) markers of myocardial damage--maximum troponin I peak--; d) angiographic characteristics--artery involved in the AMI, number of vessels with stenosis ≥70%--; and e) Killip class14--the 2 cardiologists normally responsible for the coronary unit recorded the maximum Ki-llip class during each patient's stay in hospital. The study population was divided into 2 groups according to this classification: group A--patients who did not develop heart failure (Killip class I)--, and group B--those who did develop heart failure (Killip classes II, III and IV).
Determination of Interleukin 10
A blood sample was taken from each patient within 24 h of admission (median, 18 h; range, 6-24 h) for the determination of IL-10 concentrations. Serum was obtained by centrifugation and frozen in Eppendorf tubes before transport to the laboratory for analysis. Interleukin 10 concentrations were determined using high sensitivity quantitative sandwich immunoenzyme analysis kits (DRG Instruments GMBH, Germany), following the manufacturer's recommendations. The detection limit was 1 pg/mL. The coefficients of variation for intra- and inter-analyses were 3.2 and 2.8% respectively.
Statistical Analysis
All calculations were made using SPSS software v.10.0 for Windows. Qualitative variables were expressed as percentages; quantitative variables were expressed as means ± standard deviation (SD). The proximity of the data to the normal distribution was analyzed using the Kolmogorov-Smirnov test. Pairs of qualitative variables were compared using the χ² test. Differences between the means of quantitative variables with normal distributions were analyzed using the Student t test.
RESULTS
Group A (Killip class I) was composed of 37 patients; group B contained 28 (15 in Killip class II, 10 in Killip class III, and 3 in Killip class IV). Table 1 shows the clinical characteristics of both groups of patients. The time elapsed between the onset of symptoms and the achievement of a TIMI score of 3 was similar in both groups (5.7±1.8 vs 5.9±2.1 h; P=.22).
The time elapsed from the beginning of the PTCA procedure to the reopening of the affected artery was also similar in both groups (29±16 vs 27±12 min; P=.58). A TIMI flow score of 3 was achieved in all patients. No significant differences were seen between the 2 groups with respect to the percentage of patients that received stents during PTCA (81% in group A vs 85% in group B; P=.61). No reinfarctions nor cerebrovascular accidents were recorded in either group.
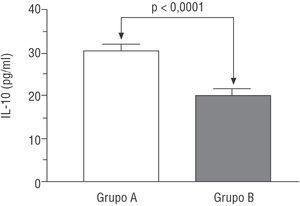
Interleukin 10 concentrations were higher in group A patients (i.e., those with AMI but who did not develop heart failure) than in group B patients (those who did develop heart failure) (30.4±10.8 vs 19.8±7.9 pg/ml; P<.001) (Figure 1).
Figure. Serum interleukin 10 concentration according to groups (group A: without heart failure; group B: with heart failure).
DISCUSSION
The development of heart failure is associated with greater mortality in patients with AMI; indeed, it is the variable most strongly associated with mortality at 28 days.15 The results of the present study indicate that patients with AMI who receive primary PTCA and who go on to develop heart failure have lower serum IL-10 concentrations in the first 24 h after the ischemic event than do those who do not develop heart failure. While it is true that no series of serum IL-10 determinations were made for each patient, the association seen between the values at admission and the development of heart failure allow one to hypothesize about their potential predictive value. Other authors have reported similar results regarding the power of IL-10 concentrations at admission to predict the later development of heart failure.16,17
Mallat et al18 reported a strong association between high IL-10 expression in atherosclerotic lesions and a reduction in the expression of inducible nitric oxide synthase, suggesting that IL-10 plays a limiting role in the inflammatory response. Smith et al19 reported that patients with unstable angina had significantly lower blood IL-10 concentrations than did those with chronic, stable angina. This suggests that lower IL-10 levels are associated with greater clinical instability, and supports the hypothesis that IL-10 plays a protective role in atherogenesis by contributing towards maintaining plaque stability, thus helping to prevent acute events.
It has been shown that the release of cytokines by the heart following AMI increases considerably after primary PTCA.20 In post post-AMI patients, the activation of proinflammatory cytokines leads to high concentrations of inducible nitric oxide synthase, nitric oxide, and peroxynitrite, all of which can have multiple harmful effects.21 In a murine ischemia-reperfusion model, Yang et al22 observed that mice with IL-10 deficiencies had an exaggerated inflammatory response in their tissues compared to wild type mice. This was manifested as an increase in neutrophil infiltration in the reperfused tissue and an increase in the production of tumor necrosis factor alpha (TNF-α), ICAM-1 adhesion molecules and the degradation products of nitric oxide. This eventually led to an increase in the size of the myocardial necrosis and in mortality in immunosuppressed animals. These findings provide evidence that endogenous IL-10 plays a protective role in myocardial ischemia and reperfusion via its inhibition of the production of TNF-α plus that of inducible nitric oxide synthase, the expression of adhesion molecules and the recruitment of neutrophils.
Acute myocardial infarction is associated with a stronger inflammatory response than is unstable angina: the latter is associated with an inflammatory mechanism unrelated to the presence of necrosis and with the extension of coronary lesions.23 In the present study, the patients who developed heart failure had lower IL-10 concentrations, which probably led to the amplification of the local inflammatory response occasioned by the ischemia- and/or reperfusion-induced damage to the myocardium.
The distributions of the proatherogenic and proinflammatory risk factors were similar in both groups of patients. It is therefore unlikely that differences in these risk factors influenced the expression of IL-10.
The present findings suggest that the potential role of IL-10 as a therapeutic agent in this clinical context should be investigated.
Limitations of the Study
The present study sample was relatively small and composed of selected patients with AMI who had undergone successful primary PTCA: the results apply, therefore, only to this kind of patient and cannot be extrapolated to other AMI reperfusion situations or to cases where reperfusion is unsuccessful. In addition, the peripheral activation of cytokines probably does not adequately reflect their tissue activation, but it is within the tissues where mediators are activated that favor ventricular remodeling, influencing the progression of disease.
The determination of a single IL-10 value for each patient may somewhat reduce the value of this work. Nevertheless, when establishing the predictive value of a variable, the values recorded in the initial assessment of a patient are the most important.24
The moment when the blood sample was taken for the determination of the IL-10 concentration differed somewhat between patients with respect to the onset of their symptoms. The results should therefore be interpreted with caution. In addition, IL-10 behavior in the post-AMI period may be influenced by certain treatments. These potential problems were not taken into account in this study. However, the conclusions drawn would probably not change: patients with a low IL-10 concentration are more likely to have a more exaggerated inflammatory response and be clinically more unstable.
CONCLUSION
In patients with AMI who undergo successful PTCA, the serum concentration of IL-10 determined in the first 24 h following the event is higher among those who do not go on to develop heart failure. This finding suggests that this anti-inflammatory cytokine exerts a protective effect on the myocardium during ischemia, reperfusion, or both.
ACKNOWLEDGEMENTS
The authors extend their thanks to the nursing personnel of our coronary unit, without whose collaboration and enthusiasm this work would not have been possible. Thanks are also due to Inés Abreu Afonso for her help in the preparation and translation of the abstract.
The interleukin kits used in this study were purchased with the financial help of Pfizer.
Correspondence: Dr. A. Domínguez Rodríguez.
La Longuera, Teide, 7, 2.a dcha. 38410 Los Realejos. Santa Cruz de Tenerife. España.
E-mail: adrvdg@hotmail.com