Acute coronary syndrome (ACS) guidelines recommend the use of newer P2Y12 inhibitors (prasugrel and ticagrelor) over clopidogrel in patients with moderate-to-high ischemic risk, unless they have an increased bleeding risk. The aim of our study was to assess the GRACE risk score and the CRUSADE bleeding risk score relative to prescription of newer P2Y12 inhibitors at discharge in ACS patients.
MethodsRetrospective analysis of a multicenter ACS registry; 3515 consecutive patients were included. The association between risk scores and prescription of newer P2Y12 inhibitors was assessed by binary logistic regression analysis.
ResultsA total of 1021 patients (29%) were treated with prasugrel or ticagrelor. On multivariate analyses, both GRACE (OR per 10 points, 0.89; 95%CI, 0.86-0.92; P < .001) and CRUSADE (OR per 10 points, 0.96; 95%CI, 0.94-0.98; P < .001) risk scores were inversely associated with the use of newer P2Y12 inhibitors. Moreover, other factors not included in these scores (revascularization approach, in-hospital stent thrombosis, major bleeding, and concomitant indication for anticoagulation therapy) also predicted the use of newer P2Y12 inhibitors.
ConclusionsNew P2Y12 inhibitors were more frequently prescribed among ACS patients with lower CRUSADE bleeding risk. However, an ischemic risk paradox was found, with higher use of these agents in patients with lower ischemic risk based on GRACE risk score estimates. These results underscore the importance of risk stratification to safely deliver optimal therapies.
Keywords
Dual antiplatelet therapy with aspirin and clopidogrel has been considered the mainstay of care in the setting of acute coronary syndrome (ACS).1 However, despite the benefits of this combination, patients continue to be at risk of further cardiovascular events2–4 which, to some extent, may be related to some inadequate antiplatelet effects of clopidogrel.5,6 This has stimulated the search for more effective antiplatelet drugs. Prasugrel and ticagrelor are newer and more potent P2Y12 inhibitors, which have been demonstrated to reduce recurrent ischemic events, albeit at the expense of increased bleeding compared with clopidogrel.7,8 Current guidelines for the management of ACS9,10 recommend the use of these new P2Y12 inhibitors over clopidogrel, mainly in patients with moderate-to-high ischemic risk, unless they have an increased risk of bleeding. Therefore, clinicians must balance risks and benefits to obtain the highest clinical net benefit of the prescribed antiplatelet therapy.
One option to assess both clinical risk as well as the potential for bleeding is the use of risk scores. In patients with ACS, the GRACE score provides the most accurate stratification of risk of death, using scores both on admission and at discharge11 and its use is recommended by current ACS guidelines.9,10 Regarding bleeding risk assessment, the CRUSADE score has been found to be the most discriminatory score to assess in-hospital major bleeding risk, mainly in patients undergoing coronary angiography.12 To date, there is no validated risk model to estimate bleeding risk after discharge in ACS patients. However, because the CRUSADE risk score performed well among patients taking dual antiplatelet therapy, this risk model may be used for bleeding risk stratification in ACS patients after hospital discharge.13 However, which risk has the higher impact or whether other factors influence clinicians’ patterns of antiplatelet selection is unknown. Therefore, the aims of this study were to assess the GRACE risk score and the CRUSADE bleeding risk score relative to prescription of new P2Y12 inhibitors (prasugrel and ticagrelor) in patients with ACS.
MethodsThe present study complied with the Declaration of Helsinki and was approved by the local ethics committee. The data analyzed in this study were obtained from a merged retrospective clinical registry including all consecutive patients with an established final diagnosis of ACS who had undergone coronary angiography in 2 tertiary hospitals from January 1, 2011 to December 31, 2015. However, for this analysis, the study population was limited to patients admitted from November 1, 2012 to December 31, 2015. This was the period when the 2 newer P2Y12 inhibitors (prasugrel and ticagrelor) were widely available in both participating hospitals, and when the prescription rate of these agents reached a relatively stable level, at around 30%. This analysis excluded early users of ticagrelor or prasugrel, who may differ from those who started these agents later (ie, eagerness to adopt new treatments or abnormal baseline risk). A total of 4653 ACS patients were recruited. Of these, 1138 were excluded (755 were admitted before November 1, 2012, 142 patients died before hospital discharge, and GRACE or CRUSADE risk scores and antiplatelet therapy at discharge were not available in 132 and 109 patients [2.8%], respectively). Therefore, the final study population consisted of 3515 hospitalized patients with ACS.
Data on demographic and clinical characteristics, complementary test results, angiographic parameters, in-hospital events, as well as treatment at discharge were collected in detail by trained cardiologists. For each patient, the GRACE 6-month mortality risk score14 and the CRUSADE bleeding risk score15 were calculated retrospectively. Patients were classified into 3 categories as a function of GRACE risk score (low risk < 100 points, intermediate risk 100-127 points, and high risk > 127 points for ST-segment elevation ACS; and low risk < 89 points, intermediate risk 89-118 points, and high risk > 118 points for non–ST-segment elevation ACS) and 5 categories as a function of CRUSADE risk score (very low risk < 21, low risk 21-30 points, moderate risk 31-40 points, high risk 41-50 points, and very high risk > 50 points). Major bleeding was defined according to the Bleeding Academic Research Consortium criteria as bleeding types 3 to 5.16 Stent thrombosis was defined following the recommendations of the Academic Research Consortium, and only definitive stent thrombosis was considered for analyses.17 The clinical management decisions about each patient were decided by the treating cardiologist and no protocol was followed to guide selection of dual antiplatelet therapy.
Categorical variables are presented as frequency values and continuous variables as mean ± standard deviation. Categorized analyses were performed according to the prescription of new P2Y12 inhibitors at discharge. Differences in baseline characteristics were compared using the Student t test for continuous variables and the chi-square test for categorical variables. To assess the independent association of GRACE and CRUSADE risk scores on new P2Y12 inhibitor prescription at discharge, we calculated odds ratios (OR) and 95% confidence intervals (95%CI) using multivariate binary logistic regression models with adjustment for other predictors of new P2Y12 inhibitor prescription at discharge. To identify variables associated with new P2Y12 inhibitor prescription at discharge, univariable binary logistic regression analyses were first performed for the all variables listed in Table 1 of the supplementary material. We next performed a stepwise backward selection binary logistic regression analysis to identify independent predictors of new P2Y12 inhibitor prescription at discharge. Covariates with P < .05 on univariable analysis and those considered of clinical interest by the investigator (diabetes mellitus) were included in the multivariate models. The entry criterion was set at P < .05, and the exit criterion was set at P = .10. To investigate the influence of the individual data elements of the CRUSADE and GRACE risk scores on new P2Y12 inhibitor prescription, we developed additional multivariate binary logistic regression models. All P values < .05 were accepted as statistically significant. Statistical analysis was performed using SPSS version 18.0 (SPSS, Inc, Chicago, Illinois, United States) and STATA version 13.0 (Stata Corp LP; Texas, United States).Table 1
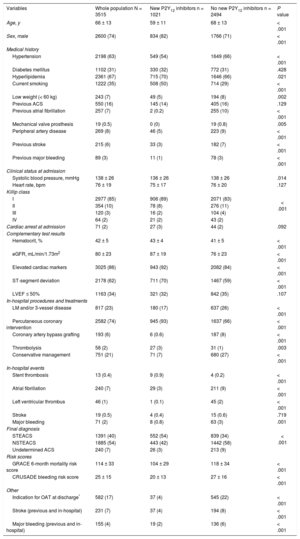
Study Population Clinical Characteristics in the Whole Population and as a Function of New P2Y12 Inhibitors Prescription at Discharge
| Variables | Whole population N = 3515 | New P2Y12 inhibitors n = 1021 | No new P2Y12 inhibitors n = 2494 | P value |
|---|---|---|---|---|
| Age, y | 66 ± 13 | 59 ± 11 | 68 ± 13 | < .001 |
| Sex, male | 2600 (74) | 834 (82) | 1766 (71) | < .001 |
| Medical history | ||||
| Hypertension | 2198 (63) | 549 (54) | 1649 (66) | < .001 |
| Diabetes mellitus | 1102 (31) | 330 (32) | 772 (31) | .428 |
| Hyperlipidemia | 2361 (67) | 715 (70) | 1646 (66) | .021 |
| Current smoking | 1222 (35) | 508 (50) | 714 (29) | < .001 |
| Low weight (< 60 kg) | 243 (7) | 49 (5) | 194 (8) | .002 |
| Previous ACS | 550 (16) | 145 (14) | 405 (16) | .129 |
| Previous atrial fibrillation | 257 (7) | 2 (0.2) | 255 (10) | < .001 |
| Mechanical valve prosthesis | 19 (0.5) | 0 (0) | 19 (0.8) | .005 |
| Peripheral artery disease | 269 (8) | 46 (5) | 223 (9) | < .001 |
| Previous stroke | 215 (6) | 33 (3) | 182 (7) | < .001 |
| Previous major bleeding | 89 (3) | 11 (1) | 78 (3) | < .001 |
| Clinical status at admission | ||||
| Systolic blood pressure, mmHg | 138 ± 26 | 136 ± 26 | 138 ± 26 | .014 |
| Heart rate, bpm | 76 ± 19 | 75 ± 17 | 76 ± 20 | .127 |
| Killip class | < .001 | |||
| I | 2977 (85) | 906 (89) | 2071 (83) | |
| II | 354 (10) | 78 (8) | 276 (11) | |
| III | 120 (3) | 16 (2) | 104 (4) | |
| IV | 64 (2) | 21 (2) | 43 (2) | |
| Cardiac arrest at admission | 71 (2) | 27 (3) | 44 (2) | .092 |
| Complementary test results | ||||
| Hematocrit, % | 42 ± 5 | 43 ± 4 | 41 ± 5 | < .001 |
| eGFR, mL/min/1.73m2 | 80 ± 23 | 87 ± 19 | 76 ± 23 | < .001 |
| Elevated cardiac markers | 3025 (86) | 943 (92) | 2082 (84) | < .001 |
| ST-segment deviation | 2178 (62) | 711 (70) | 1467 (59) | < .001 |
| LVEF ≤ 50% | 1163 (34) | 321 (32) | 842 (35) | .107 |
| In-hospital procedures and treatments | ||||
| LM and/or 3-vessel disease | 817 (23) | 180 (17) | 637 (26) | < .001 |
| Percutaneous coronary intervention | 2582 (74) | 945 (93) | 1637 (66) | < .001 |
| Coronary artery bypass grafting | 193 (6) | 6 (0.6) | 187 (8) | < .001 |
| Thrombolysis | 58 (2) | 27 (3) | 31 (1) | .003 |
| Conservative management | 751 (21) | 71 (7) | 680 (27) | < .001 |
| In-hospital events | ||||
| Stent thrombosis | 13 (0.4) | 9 (0.9) | 4 (0.2) | < .001 |
| Atrial fibrillation | 240 (7) | 29 (3) | 211 (9) | < .001 |
| Left ventricular thrombus | 46 (1) | 1 (0.1) | 45 (2) | < .001 |
| Stroke | 19 (0.5) | 4 (0.4) | 15 (0.6) | .719 |
| Major bleeding | 71 (2) | 8 (0.8) | 63 (3) | .001 |
| Final diagnosis | < .001 | |||
| STEACS | 1391 (40) | 552 (54) | 839 (34) | |
| NSTEACS | 1885 (54) | 443 (42) | 1442 (58) | |
| Undetermined ACS | 240 (7) | 26 (3) | 213 (9) | |
| Risk scores | ||||
| GRACE 6-month mortality risk score | 114 ± 33 | 104 ± 29 | 118 ± 34 | < .001 |
| CRUSADE bleeding risk score | 25 ± 15 | 20 ± 13 | 27 ± 16 | < .001 |
| Other | ||||
| Indication for OAT at discharge* | 582 (17) | 37 (4) | 545 (22) | < .001 |
| Stroke (previous and in-hospital) | 231 (7) | 37 (4) | 194 (8) | < .001 |
| Major bleeding (previous and in-hospital) | 155 (4) | 19 (2) | 136 (6) | < .001 |
ACS, acute coronary syndrome; eGFR, estimated glomerular filtration rate; LM, left main artery; LVEF, left ventricular ejection function; NSTEACS, non–ST-segment elevation acute coronary syndrome; OAT, oral anticoagulation therapy; STEACS, ST-segment elevation acute coronary syndrome.
Data are expressed as mean ± standard deviation or No. (%).
The study population consisted of 3515 hospitalized patients with ACS. A total of 1021 patients (29%) were treated with new P2Y12 inhibitors at discharge. Prasugrel was used in 346 (9.8%) and ticagrelor in 675 (19.2%).
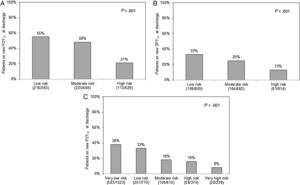
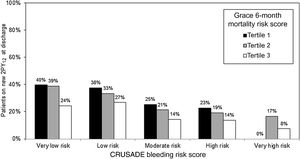
Table 1 shows the study population characteristics according to new P2Y12 prescription at discharge. Patients on new P2Y12 inhibitors had lower GRACE (104 ± 29 vs 118 ± 34; P < .001) and CRUSADE (20 ± 13 vs 27 ± 16; P < .001) scores. A stepwise decrease in the proportion of patients on new P2Y12 inhibitors was seen with increasing bleeding and ischemic risk categories (Figure 1). This underuse of new P2Y12 inhibitors with increasing ischemic risk was seen in all bleeding risk categories (Figure 2). Thus, the higher the ischemic or bleeding risk, the lower the rate of new P2Y12 inhibitor prescription.
Rate of new P2Y12 inhibitor prescription at discharge according to GRACE 6-month mortality risk categories in patients presenting with STEACS (A) or NSTEACS (B) and CRUSADE bleeding risk categories (C). STEACS, ST-segment elevation acute coronary syndrome; NSTEACS, non–ST-segment elevation acute coronary syndrome.
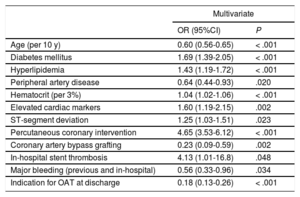
In multivariate binary logistic regression analyses, we found both GRACE (OR per 10 points, 0.89; 95%CI, 0.86-0.92; P < .001) and CRUSADE (OR per 10 points, 0.96; 95%CI, 0.94-0.98; P < .001) risk scores to be associated with new P2Y12 inhibitor prescription at discharge (Figure 3). This inverse association with GRACE and CRUSADE risk scores persisted when prasugrel and ticagrelor prescription was analyzed separately (prasugrel = OR per 10 points in the GRACE risk score, 0.86; 95%CI, 0.82-0.90; P < .001 and OR per 10 points in the CRUSADE risk score, 0.95; 95%CI, 0.92-0.99; P = .009; ticagrelor = OR per 10 points in the GRACE risk score, 0.91; 95%CI, 0.88-0.94; P < .001 and OR per 10 points in the CRUSADE risk score, 0.96; 95%CI, 0.94-0.99; P = .003). Detailed data on these multivariate analyses are described in Table 2A of the supplementary material and Table 2B of the supplementary material. As shown in Table 2, when the individual data elements of both risk scores were included in a single multivariate model, age (OR per 10 years, 0.60; 95%CI, 0.56-0.65; P < .001), diabetes mellitus (OR, 1.69; 95%CI, 1.39-2.05; P < .001), peripheral artery disease (OR, 0.64; 95%CI, 0.44-0.93; P < .020), hematocrit level (OR per 3%, 1.04; 95%CI, 1.02-1.06; P <.001), elevated cardiac markers (OR; 1.60; 95%CI, 1.19-2.15; P = .002) and ST-segment deviation (OR, 1.25; 95%CI, 1.03-1.51; P = .023) remained as predictors of new P2Y12 inhibitor prescription at discharge. Other independent predictors, not included in the GRACE or CRUSADE risk scores, were hyperlipidemia, percutaneous coronary intervention, coronary artery bypass grafting, in-hospital stent thrombosis, major bleeding (previous or in-hospital), and indication for oral anticoagulation therapy at discharge (Table 2).
Multivariate odds ratios for the association between GRACE and CRUSADE risk scores and new P2Y12 inhibitor prescription at discharge. 95%CI, 95% confidence interval; OR, odds ratio. For variables included in multivariate models, see Table 2A of the supplementary material and Table 2B of the supplementary material.
Multivariate Logistic Regression Analysis Including Individual Data Elements of GRACE and CRUSADE Risk Scores for Predicting new P2Y12 Inhibitors Prescription at Discharge
| Multivariate | ||
|---|---|---|
| OR (95%CI) | P | |
| Age (per 10 y) | 0.60 (0.56-0.65) | < .001 |
| Diabetes mellitus | 1.69 (1.39-2.05) | < .001 |
| Hyperlipidemia | 1.43 (1.19-1.72) | < .001 |
| Peripheral artery disease | 0.64 (0.44-0.93) | .020 |
| Hematocrit (per 3%) | 1.04 (1.02-1.06) | < .001 |
| Elevated cardiac markers | 1.60 (1.19-2.15) | .002 |
| ST-segment deviation | 1.25 (1.03-1.51) | .023 |
| Percutaneous coronary intervention | 4.65 (3.53-6.12) | < .001 |
| Coronary artery bypass grafting | 0.23 (0.09-0.59) | .002 |
| In-hospital stent thrombosis | 4.13 (1.01-16.8) | .048 |
| Major bleeding (previous and in-hospital) | 0.56 (0.33-0.96) | .034 |
| Indication for OAT at discharge | 0.18 (0.13-0.26) | < .001 |
95%CI, 95% confidence interval; ACS acute coronary syndrome; LM, left main artery; LVEF, left ventricular ejection function; OAT, oral anticoagulation therapy; OR, odds ratio.
The multivariate model included individual data elements of GRACE and CRUSADE risk scores, hypertension, hyperlipidemia, current smoking, low weight < 60kg, previous ACS, LVEF ≤ 50%, LM and/or 3-vessel disease, percutaneous coronary intervention, coronary artery bypass grafting, conservative management, in-hospital stent thrombosis, final ACS diagnosis, indications for OAT at discharge (atrial fibrillation [previous or in-hospital], mechanical valve prosthesis, left ventricular thrombus and other), stroke (previous and in-hospital) and major bleeding (previous and in-hospital).
In this study, we evaluated prescription of newer, more potent P2Y12 inhibitors at discharge relative to clinical risk and risk for bleeding in patients with ACS. We found that a perceived high bleeding risk led to lower prescription of these agents, despite generally higher risk in such patients. This finding is in accordance with current guidelines that do not support the use of prasugrel or ticagrelor in patients at high risk of bleeding.9,10 In contrast, current guidelines also recommend the use of prasugrel and ticagrelor in patients at moderate-to-high risk of ischemic events,9,10 due to the reduction in ischemic events with both new P2Y12 inhibitors.7,8 Furthermore, some authors have proposed specific protocols for the selection of antiplatelet therapy in patients with ACS in which the GRACE risk score is the first discriminative criteria (clopidogrel if GRACE < 109 and prasugrel or ticagrelor if GRACE ≥ 109 and no contraindications or high bleeding risk).18 However, in our study, we found that higher ischemic risk based on GRACE risk score was not related to prescription of these agents. Indeed, the higher the estimated ischemic risk, the lower the rate of new P2Y12 inhibitor prescription at discharge. Specifically, higher age was the only ischemic risk factor included in the GRACE risk score independently associated with lower prescription of novel P2Y12 inhibitors. This is not surprising, given that advanced age is a recognized bleeding risk factor.19 Our findings concur with those of previous studies reporting underuse of prasugrel or ticagrelor in older patients.20 Although not included in the CRUSADE risk score, many other bleeding risk scores developed for ACS patients include age to estimate bleeding risk.21–23 Notably, we found that patients with diabetes were more likely to receive the newer P2Y12 agents, even though diabetes tends to be associated with a higher incidence of bleeding events.24 This prescribing behavior may be explained by the accumulating evidence for a net clinical benefit with new P2Y12 inhibitors in diabetic patients following ACS.25 This enhanced benefit in patients with diabetes may be due to an impaired response to clopidogrel in such patients26 together with their heightened risk for ischemic events.27 Moreover, we found other predictors of novel P2Y12 prescription not included in the aforementioned scores. All of them were predictable, such as previous PCI as well as stent thrombosis (in both cases, more potent P2Y12 inhibitor use is now widely accepted) or the concomitant indication of oral anticoagulation therapy at discharge (in this scenario, current guidelines do not recommend the use of new P2Y12 inhibitors9,10). In the case of dyslipidemia, we did not find an obvious explanation, beyond its role as a cardiovascular risk factor, and it could be a hazardous association.
We propose several reasons to explain the lower prescription of new P2Y12 inhibitors among patients with higher GRACE risk scores: a) the fear of bleeding complications due to the difficult separation between ischemic and bleeding risk in ACS patients because of the high correlation between the 2 scores.28 In this line, our group has previously found that GRACE risk score is not inferior to the CRUSADE risk score in predicting in-hospital major bleeding29; b) the phenomenon known as the risk treatment paradox, which postulates that clinicians are more likely to treat ACS based on guidelines in low risk patients30; c) the perceived high risk of bleeding in older patients and the strong influence of age in the GRACE risk score; and d) the existence of other factors not included in the GRACE risk score that influence clinicians’ patterns of antiplatelet selection.
The main implication of our study is that it reinforces the importance of appropriate risk assessment, which is an important issue for therapeutic decision-making. There is strong evidence demonstrating that an aggressive treatment approach has the potential to change the prognosis of patients with ACS, although this effect is frequently risk dependent.31,32 Currently, it is accepted that high risk ischemic patients with ACS deserve more aggressive management, including more potent antithrombotic treatment and a rapid invasive strategy, while lower risk patients may do well with less potent antithrombotic treatment and a more selective invasive strategy.9,10 The accumulated evidence supports that more potent antithrombotic drugs and invasive procedures can reduce the number of ischemic events in ACS patients, but these treatments usually increase the risk of bleeding.33 Therefore, both ischemic and bleeding risk scores have become necessary in ACS risk assessment for the calculation of trade-offs between ischemic risk reduction and spontaneous and treatment-related bleeding hazards. The risk paradox we have identified (with lower risk patients being treated more aggressively than higher risk patients) may be partly driven by perceived bleeding risk. How the risks may be balanced may be facilitated by tools with even better discrimination for bleeding and/or ischemic complications. Future studies investigating the impact on patient outcomes by integrating both risks are needed to resolve this important issue.
LimitationsOur study has the inherent limitations of being a retrospective study that included ACS patients undergoing coronary angiography during the index hospitalization in 2 Spanish tertiary hospitals. Therefore, the applicability of the present results should be viewed with caution in centers with other types of patients and medical facilities, and should be considered as hypothesis-generating. The inclusion of patients with ACS undergoing coronary angiography may cause selection bias and favor a high proportion of patients with ST-segment elevation ACS, who are more frequently managed invasively. Moreover, we had no data on socioeconomic status, education, employment situation, or nationality, which may influence newer antiplatelet prescription. Last, although we analyzed the impact of in-hospital events on treatment at discharge, we have no information on the relationship between these events and previous antithrombotic therapy or its modifications.
ConclusionsIn conclusion, our study results suggest that several factors influence clinicians when prescribing new P2Y12 at discharge in ACS patients, with an appropriate underuse of these agents in patients at high bleeding risk. This led to an ischemic risk paradox, however, illustrating the complexity of operationalizing clinical practice guidelines in real-world settings. Understanding new antiplatelet prescribing behavior in such settings is crucial to inform interpretation of new studies exploring the benefits of new antiplatelet drugs in ACS, and to identify opportunities for future efforts to improve prescription quality.
CONFLICTS OF INTERESTE. Abu-Assi is Associate Editor of Revista Española de Cardiología.
- –
New P2Y12 inhibitors (prasugrel and ticagrelor) are recommended by current ACS guidelines in patients with moderate-to-high ischemic risk, unless they have an increased bleeding risk. These guidelines also recommend the GRACE and CRUSADE risk scores for ischemic and bleeding risk assessment, respectively. However, which risk has the stronger impact or whether other factors influence clinicians’ patterns of antiplatelet selection is unknown.
- –
In this study, we found an appropriate use of new P2Y12 inhibitors among patients at high bleeding risk. However, an ischemic risk paradox was found, with higher use of these agents in patients with lower ischemic risk based on GRACE risk score. Moreover, we found that other factors, beyond these scores, influenced clinicians’ prescription patterns. This illustrates the complexity of operationalizing clinical practice guidelines in real-world settings and reinforces the importance of appropriate risk assessment for therapeutic decision-making.