Acetylsalicylic acid, synthesized in an industrial environment in 1897, was introduced to the market as Aspirin® in 1899. For about 70 years it represented the mainstay of analgesic/antiinflammatory drug therapy and its pharmacologic actions provided the template for the synthesis of novel nonsteroidal antiinflammatory drugs. Following several fundamental discoveries on its mechanism of action as an antiplatelet drug in the seventies, aspirin has lived a second life as an antithrombotic agent, becoming a fundamental component of cardiovascular prevention and treatment.1 Making the jump from a largely over-the-counter analgesic remedy to a life-saving prescription drug represents a success story of independent translational research. Key components of success were: a) mechanistic insight into the way in which aspirin inhibits platelet function; b) careful studies of the clinical pharmacology of its antiplatelet effect, establishing the unusual requirements of low dose and long dosing interval for optimal platelet inhibition; and c) a large number of adequately sized, placebo-controlled clinical trials to demonstrate its efficacy and safety in a variety of clinical settings characterized by high cardiovascular risk.2
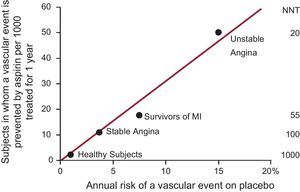
As with other cardiovascular prevention strategies (eg, blood pressure- or lipid-lowering drugs), low-dose aspirin can only reduce a fraction (about one quarter) of all major vascular events, not because of “resistance” to its antiplatelet effect, but because of the multifactorial nature of atherothrombosis.3 As with statins or antihypertensive drugs, the absolute benefits of aspirin (how many vascular events can be prevented by treating 1000 patients for one year) are linearly related to the underlying cardiovascular risk of the patients (Fig. 1).4
The absolute risk of vascular complications is the major determinant of the absolute benefit of antiplatelet prophylaxis. Data are plotted from placebo-controlled aspirin trials in different clinical settings. For each category of patients, the abscissa denotes the absolute risk of experiencing a major vascular event as recorded in the placebo arm of the trial(s). The absolute benefit of antiplatelet treatment is reported on the ordinate as the number of subjects in whom an important vascular event (nonfatal myocardial infarction, nonfatal stroke, or vascular death) is prevented by treating 1000 subjects with aspirin for 1 year. Numbers needed to treat to prevent 1 event in each clinical setting are also displayed on the right hand side of the figure. MI, myocardial infarction; NNT, numbers needed to treat. Modified with permission from Patrono et al.4
An extensive literature has described lower-than-expected inhibition of platelet function (often referred to as “resistance”) in a variable proportion of aspirin-treated patients.4 However, neither the mechanism(s) of response variability nor its reversibility have been established. Major limitations of previous studies are related to: a) inadequate ascertainment of compliance; b) largely undefined time interval between aspirin dosing and measurement of platelet inhibition; c) dichotomous definition of responder vs nonresponder status based on a single determination of platelet function using arbitrary thresholds of response; and d) lack of intervention studies to clarify the underlying mechanism(s) of variability in aspirin responsiveness.
To overcome these limitations, Rocca et al. have recently developed an investigative approach based on the following innovative features: a) a witnessed drug administration; b) accurate timing of blood sampling at 12, 15, 18, 21, and 24h after dosing in order to assess the maximal level of platelet inhibition and the kinetics of its reversal; c) use of a mechanism-based biochemical end-point, ie, serum thromboxane B2 (TXB2), with the highest specificity and sensitivity to monitor aspirin pharmacodynamics; and d) randomized intervention studies to test the reproducibility of the abnormal biochemical phenotype, and its potential reversal by increasing the aspirin dose or shortening its dosing interval.5,6
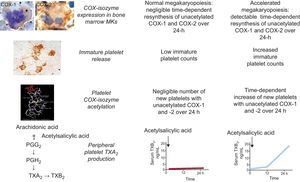
These studies have characterized substantial interindividual variability in the recovery rate of platelet cyclooxygenase (COX)-1 activity during the 12 to 24h dosing interval of aspirin administration in well controlled type 2 diabetic patients as well as in patients without diabetes, with an indication for antiplatelet therapy for primary or secondary prevention.5 While very profound suppression of platelet thromboxane production was measurable in the vast majority of patients at 12h after a witnessed administration of aspirin, a variable linear increase in serum TXB2 production between 12 and 24h was unraveled by repeated determinations.5 While diabetic patients with the steepest COX-1 recovery slope showed significantly higher mean platelet volume and body mass index, higher body weight was the only independent predictor of a faster recovery in nondiabetics.5 This abnormal biochemical phenotype was relatively stable, and could be completely reversed by aspirin 100mg given twice daily.5 Thus, the variable turnover rate of the drug target (ie, platelet COX-1) appears to represent the main mechanism contributing to the interindividual variability in drug response. Using a similar methodological approach, we recently demonstrated that the abnormal megakaryopoiesis which characterizes essential thrombocythemia is responsible for a shorter-lasting antiplatelet effect of low-dose aspirin through faster renewal of unacetylated platelet COX-1 (Fig. 2), and impaired platelet inhibition can be rescued by modulating the aspirin dosing interval rather than the dose.6
Model of altered aspirin pharmacodynamics in essential thrombocytemia. Under conditions of normal megakaryopoiesis, low-dose aspirin acetylates cyclooxygenase (COX) isozymes in both circulating platelets and bone marrow megakaryocytes (MKs), but negligible amounts of unacetylated enzymes are resynthesized within the 24-h dosing interval. This pharmacodynamic pattern is associated with virtually complete suppression of platelet thromboxane (TX)A2 production in the peripheral blood throughout the dosing interval. Under conditions of abnormal megakaryopoiesis, an accelerated rate of COX-isozyme resynthesis is biologically plausible in bone marrow MKs, accompanied by faster release of immature platelets with unacetylated enzyme(s) during the aspirin dosing interval, and in particular between 12 and 24h after dosing. This pharmacodynamic pattern is associated with incomplete suppression of platelet TXA2 production in the peripheral blood and time-dependent recovery of TXA2-dependent platelet function during the 24-h dosing interval. Immunohistochemistry panels depict MKs from an ET patient stained for COX-1 and from a normal subject stained for COX-2 and peripheral washed platelets from an ET patient stained for COX-2. PG, prostaglandin. Reproduced with permission from Pascale et al.6
Based on large-scale observational studies, low-dose aspirin approximately doubles the risk of major extracranial bleeds, particularly upper gastrointestinal hemorrhage.7 In the meta-analysis of individual participant data from 6 primary prevention trials performed by the Antithrombotic Trialists’ Collaboration,8 aspirin increased major gastrointestinal and other extracranial bleeds by about half (0.10% vs 0.07% per year; relative risk, 1.54; 95% confidence interval, 1.30-1.82; P<.0001). Major bleeds were recorded in only 5 of the 16 secondary prevention trials, so a meta-analysis might be unreliable. There was again, however, a significant excess of major bleeds among those taking aspirin (relative risk, 2.69; 95% confidence interval, 1.25-5.76; P=.01), with no significant heterogeneity between the relative risks in the 6 primary and these 5 secondary prevention trials.8 Interestingly, the main risk factors for coronary events, including diabetes mellitus, were also associated with hemorrhagic events, although for most the associations were slightly weaker for bleeding than for occlusive events.8
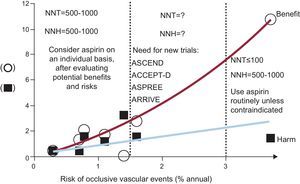
The benefit/risk profile of low-dose aspirin can vary substantially over the cardiovascular risk continuum, from an area of high risk where benefits clearly outweigh the excess of major bleeding complications to an area of low risk where the number of vascular events avoided equals the number of major bleeds caused by aspirin (Fig. 3)9–15. While the evidence from randomized clinical trials in these areas of the cardiovascular risk continuum is pretty straightforward and forms the basis of current treatment guidelines and recommendations,16 there is an area of intermediate risk where we clearly need new trials. At least 4 randomized trials are currently ongoing in about 40 000 subjects considered to be at enhanced cardiovascular risk because of diabetes mellitus (ASCEND17 and ACCEPT-D18), advanced age (ASPREE19), or a cluster of risk factors that do not include diabetes (ARRIVE20).
Benefits and risks of low-dose aspirin in primary-prevention trials. The numbers of vascular events avoided (circles) and episodes of major bleeding (squares) caused per 1000 patients treated with aspirin per year are plotted from the results of 6 individual placebo-controlled trials of aspirin in different populations characterized by various degrees of cardiovascular risk, as noted on the abscissa: Women's Health Study,9 Physicians’ Health Study,10 Primary Prevention Project,11 Hypertension Optimal Treatment Study,12 British Doctors Trial,13 and Thrombosis Prevention Trial,14 and one trial in patients with chronic stable angina, the Swedish Angina Pectoris Aspirin Trial.15. The calculated number needed to treat (NNT) and number needed to harm (NNH) are also shown. Modified with permission from Patrono et al.2
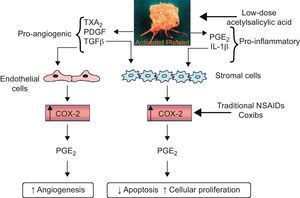
Finally, there is a very large body of evidence suggesting that aspirin may interfere with the early stages of neoplastic transformation of a normal intestinal epithelium (particularly in the colorectal section) towards a sporadic adenoma and its progression to cancer.21 The recent findings of reduced incidence and mortality due to colorectal cancer in people exposed to once daily regimens of aspirin treatment, and the apparent saturability of the chemopreventive effect at low-doses,22–24 have raised the intriguing possibility that this effect may be related to downregulation of signaling events generated by platelet activation at sites of intestinal mucosal injury21,25 (Fig. 4).
Hypothesized mechanism by which the inhibition of COX–1 in platelets by low-dose aspirin may suppress the induction of COX–2 in adjacent nucleated cells of the intestinal mucosa in early-stage neoplasia. Platelet activation at sites of intestinal mucosal injury might trigger downstream signaling events leading to reduced apoptosis, enhanced cellular proliferation and angiogenesis. The hypothesized mechanism by which the inhibition of COX–1 in platelets by low-dose aspirin may suppress the induction of COX–2 in adjacent nucleated cells of the intestinal mucosa in early-stage neoplasia is depicted. The sequential involvement of COX–1 and COX–2 would explain the similar inhibitory effects of deletion of either gene in murine intestinal tumorigenesis, as well as the similar effects of low-dose aspirin and coxibs in preventing sporadic colorectal adenoma recurrence in humans. COX, cyclooxygenase; IL, interleukin; NSAIDs: nonsteroidal antiinflammatory agents; PDGF, platelet-derived growth factor; PGE2, prostaglandin E2; TGFβ, transforming growth factor-β; TX, thromboxane. Reproduced with permission from Thun et al.21
Following the successful translational paradigm that led to the development of low-dose aspirin as an antithrombotic agent, additional joint efforts of academia and industry are urgently needed in order to understand its mechanism of action as a chemopreventive agent, define the optimal dose and dosing regimen to achieve this effect, and establish its efficacy and safety in cancer prevention. It has been argued that even a 10% reduction in overall cancer incidence from prophylactic aspirin treatment would tilt the balance of benefits and risks, and substantially broaden the indication for treatment in populations at average risk.21,26
FUNDINGThe author's studies were supported by grants from the European Commission (EICOSANOX Integrated Project 005033), the European Union's Seventh Framework Program (FP7/2007-2013) for the Innovative Medicine Initiative under grant agreement n° IMI/115006 (the SUMMIT consortium), and Bayer AG.
CONFLICTS OF INTERESTProf. Patrono has received consulting and lecture fees from AstraZeneca, Bayer AG, Eli Lilly and Merck.
The expert editorial assistance of Mrs. Daniela Basilico is gratefully acknowledged.
.