Coronary bifurcation lesions can be approached using a simple or a complex strategy. In clinical trials with first-generation drug-eluting stents, the complex strategy was not superior to the simple approach. However, to date, the best strategy when using second-generation drug-eluting stents has not been defined.
MethodsWe performed a prospective randomized study comparing a simple vs a complex strategy involving T-stenting for the percutaneous revascularization of bifurcation lesions using the everolimus-eluting stent. Angiographic and clinical follow-up were performed at 9 months.
ResultsWe included 70 lesions in 69 patients, who were randomized to the simple (34 lesions, 33 patients) or complex strategy (36 lesions and patients). In all, 85.6% of the lesions included were true bifurcations. The crossover rate was 17.1%. The binary restenosis rate was 12.1%, with no differences between the groups. Side branch restenosis tended to be higher with the simple strategy in the intention to treat analysis (10.7% vs 0%) but not in the per protocol analysis (5.9% vs 4.2%). The incidence of major adverse cardiac events (cardiac death, myocardial infarction, and target vessel revascularization) was 9.2%, with no differences between groups. There were no cases of stent thrombosis.
ConclusionsAccording to the clinical and angiographic findings, the complex strategy was not significantly superior to the simple approach in the revascularization of bifurcation lesions with second-generation everolimus-drug eluting stents.
Keywords
Percutaneous revascularization of bifurcation lesions continues to be a challenge that attracts a great deal of research. A large part of this effort has focused on determining the best technique for the treatment of bifurcation lesions, comparing the simple strategy (stent implantation only in the main vessel) and the complex approach (stents in the main vessel and side branch).
In contrast to other scenarios, the initial data on drug-eluting stents (DES) in the treatment of bifurcation lesions were unsatisfactory, especially due to the high rates of side branch restenosis and of stent thrombosis.1 Subsequently, better clinical and angiographic results were achieved in new studies that focused greater attention on technical issues, especially adequate coverage of the side branch in the complex strategy. However, the hypothesis that DES implantation in the main vessel and side branch would improve the results of the simple approach has yet to be confirmed, regardless of the modality employed in the complex approach.2–7
Until now, the trials undertaken to compare the simple and complex strategies for the treatment of bifurcation lesions have been performed with first-generation DES. Consequently, it is not known whether the use of second-generation DES, which have greater efficacy and safety, could lead to different conclusions. Among the second-generation DES, the everolimus-eluting stent (EES) offers important advantages in the treatment of bifurcation lesions, especially with regard to safety.8
Our study was designed to compare 2 strategies for the revascularization of bifurcation lesions, simple and complex with T-stenting, in which the EES was systematically used.
METHODSStudy Design. Patient InclusionThis was a prospective, randomized, open study in which bifurcation lesions were assigned to 1 of 2 arms of interventional management with EES: stent placement in the main branch (simple strategy) and stent placement in main branch and side branch using the T-stenting technique (complex strategy). The study was registered in ClinicalTrials.gov with the identifier NCT00916695; it was originally intended to be a multicenter trial, but given the small number of patients enrolled, we decided to concentrate it in a single center to avoid dispersion of the trial and achieve a homogeneous study population. The study was approved by the ethics committee of our center and of the centers that had considered participating. The enrollment period was from July 2009 to March 2011. All the patients signed an informed consent form.
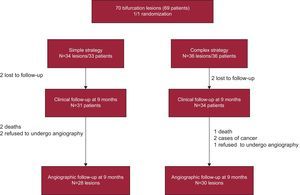
A flow chart of the study is shown in the Figure. We proposed consecutive inclusion of patients with lesions considered to be true bifurcation lesions on visual estimation (involvement of main vessel and side branch), with a diameter of 2.5mm to 4mm in the main vessel and greater than 2mm in the side branch. The exclusion criteria were left main coronary artery disease, thrombotic lesions, acute coronary syndrome within the preceding 48h, severe ventricular dysfunction (ejection fraction less than 30%), and severe renal failure (creatinine>3mg/dL).
Block randomization was carried out in random blocks of 4, with a computer-generated centralized substitution list and a code list created specifically for our center; masking was carried out by placing the randomization list in the safekeeping of the principal investigator, who informed the operator of the arm to which each intervention was allocated.
Stent RevascularizationThe study involved implantation of the Xience Prime stent (Abbott Vascular; United States), a second-generation DES composed of a MULTI-LINK 8 cobalt-chromium platform in which the drug everolimus is delivered by means of a fluoridated biocompatible copolymer.
The patients were pretreated with acetylsalicylic acid; those who were not taking clopidogrel prior to the procedure received a 600-mg loading dose of this agent immediately afterward.
Four expert interventional cardiologists from the Endovascular Unit of Hospital Virgen Macarena in Seville, Spain, performed all the procedures following the same technical guidelines.
The simple strategy involved provisional T-stenting in accordance with the following recommendations: serial predilatation of the main vessel and side branch, and subsequent implantation of the EES in the main vessel. When the outcome in the side branch was suboptimal (Thrombolysis in Myocardial Infarction grade<3, ostial lesion>75%), it was left to the discretion of the operator whether to repeat balloon dilatation or to undertake implantation of a second EES to form a “T”.
When the complex strategy was used, only the T-stenting technique was employed: following initial predilatation in both vessels, an EES was implanted in the main vessel; subsequently, the stent was recrossed to access the side branch, where an EES was implanted in a position in which it protruded as little as possible into the main vessel.
For both strategies, the decision to carry out postdilatation by means of simultaneous inflation of balloons in the main vessel and side branch (kissing balloon) was left to the discretion of the operator.
The procedure was considered successful when it was completed without incidents and the angiographic results were satisfactory (residual stenosis in the main vessel and side branch<50%).
Other lesions in the main vessels could also be treated during the same procedure, with the recommendation that these procedures be undertaken after treatment of the bifurcation lesion targeted in this study and that the Xience Prime stent be used.
Dual antiplatelet therapy was recommended for the first year following the intervention.
Follow-upClinical follow-up (valid when carried out by telephone) and follow-up angiography were scheduled for 9 months postintervention.
During the clinical follow-up, we recorded the occurrence of cardiac death, myocardial infarction (defined as hospital admission with a diagnosis of acute coronary syndrome with or without ST segment elevation), repeat revascularization of the target vessel, and stent thrombosis (definite or probable9).
In this study, we considered both ischemia-driven revascularization of the target vessel and revascularization indicated by the operator during angiographic follow-up.
Angiographic EvaluationBifurcation lesions were classified according to the definition of Medina et al.,10 the lesions were studied angiographically at 3 time points: baseline, immediately after the intervention, and 9 months later. Prior to selective angiography, 0.2mg of nitroglycerine were administered via the coronary artery.
The angiographic studies were quantitatively evaluated by a single operator, who was especially trained to perform this task by personnel from Medis, using a software package specific for bifurcation lesions (QAngio XA 7.2, Medis Medical Imaging Systems; The Netherlands). The quantitative analysis carried out with this system involved 3 segments: the proximal and distal main vessel and the side branch. Following revascularization, quantification was performed in the segment of the main vessel between the margins (±5mm) of the stent-treated portion; in the side branch, we quantified the margins of the stent or, in the absence of the stent, the portion located approximately 10mm from the ostium of that branch. The same projections were selected for quantification at all 3 time points.
Study End PointsThe primary endpoint was comparison of the simple and the complex strategies in terms of the rate of angiographic binary restenosis (obstruction>50%) in the treated bifurcation lesion (main vessel and/or side branch) 9 months after revascularization.
There were 2 secondary endpoints: one clinical, consisting of the rate of adverse cardiac events after 9 months of follow-up (cardiac death, myocardial infarction, revascularization of the target vessel); and the other angiographic, concerning the percentage of angiographic restenosis (>50%) in the side branch.
Statistical AnalysisBoth intention to treat and per protocol (treatment actually carried out) analyses were performed.
The study was designed to evaluate the difference between 2 techniques in terms of the revascularization rate of bifurcation lesions. To do this, the sample size was predetermined; considering a predicted difference in the rate of angiographic binary restenosis in the bifurcation lesions of 11%,11 with a level of significance of 5%, a statistical power of 80%, and using 2-tailed Fisher's exact test, the sample size needed to show this difference would be 131 patients/lesions per group. The final study sample consisted of 70 lesions in 69 patients, which reduced the power of the study to 45%.
Descriptive statistical analysis revealed the absolute and relative incidence, expressed as number and percentage. For continuous variables, we first applied a goodness-of-fit test after confirmation of the normal distribution by means of the Kolmogorov-Smirnov test.
Differences between the simple and complex strategies were analyzed using Pearson's chi-square test for categorical variables and using Student's t test for independent samples for continuous variables with normal distribution. In all the analyses, we considered a statistically significant safety level of at least 95% (P<.05).
All of the analyses were carried out using the Statistical Package for the Social Sciences (SPSS, version 19.0) software package (SPSS, Inc.; Chicago, Illinois, United States).
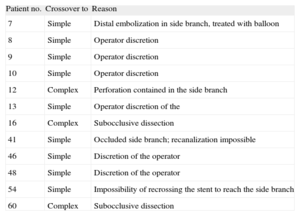
RESULTSInclusion, Crossover From One Strategy to Another, and Rate of Follow-upA total of 70 lesions (69 patients) were included; 34 lesions were randomized to the simple strategy group and 36 to the complex group. In the complex strategy group, there were 9 cases of crossover to the simple strategy, while 3 lesions allocated to the simple strategy group were treated with the complex strategy (total crossover rate, 17.1%) (Table 1).
Crossover and the Underlying Reasons
| Patient no. | Crossover to | Reason |
| 7 | Simple | Distal embolization in side branch, treated with balloon |
| 8 | Simple | Operator discretion |
| 9 | Simple | Operator discretion |
| 10 | Simple | Operator discretion |
| 12 | Complex | Perforation contained in the side branch |
| 13 | Simple | Operator discretion of the |
| 16 | Complex | Subocclusive dissection |
| 41 | Simple | Occluded side branch; recanalization impossible |
| 46 | Simple | Discretion of the operator |
| 48 | Simple | Discretion of the operator |
| 54 | Simple | Impossibility of recrossing the stent to reach the side branch |
| 60 | Complex | Subocclusive dissection |
After 9 months, 65 patients underwent clinical follow-up: 31 (93.9%) of those in the simple strategy group and 34 (94.4%) of those who had been treated with the complex strategy; angiographic follow-up was performed in 58 lesions: 28 (82.3%) in the simple strategy group and 30 (83.3%) in the complex strategy group (Figure).
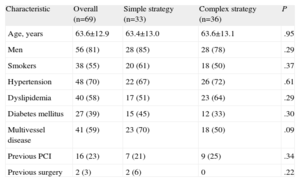
Baseline Clinical CharacteristicsThe baseline clinical characteristics of the 2 groups are shown in Table 2. Although the differences were not statistically significant, the population allocated to the simple strategy tended to have higher rates of diabetes mellitus (45% vs 33%) and multivessel disease (70% vs 50%). The population was mostly male with a high prevalence of cardiovascular risk factors and one-fourth had a history of coronary revascularization.
Baseline Clinical Data of All the Patients, Distributed According to Strategy in the Intention to Treat Analysis
| Characteristic | Overall (n=69) | Simple strategy (n=33) | Complex strategy (n=36) | P |
| Age, years | 63.6±12.9 | 63.4±13.0 | 63.6±13.1 | .95 |
| Men | 56 (81) | 28 (85) | 28 (78) | .29 |
| Smokers | 38 (55) | 20 (61) | 18 (50) | .37 |
| Hypertension | 48 (70) | 22 (67) | 26 (72) | .61 |
| Dyslipidemia | 40 (58) | 17 (51) | 23 (64) | .29 |
| Diabetes mellitus | 27 (39) | 15 (45) | 12 (33) | .30 |
| Multivessel disease | 41 (59) | 23 (70) | 18 (50) | .09 |
| Previous PCI | 16 (23) | 7 (21) | 9 (25) | .34 |
| Previous surgery | 2 (3) | 2 (6) | 0 | .22 |
PCI, percutaneous coronary intervention.
The data are expressed as no. (%) or mean±standard deviation.
Among the clinical characteristics of the patients who underwent clinical/angiographic follow-up vs those who did not, differences were found only in hypertension (75% vs 42%, respectively; P=.036) and multivessel disease (56% vs 75%); these differences were significant only in hypertension. In the remaining variables studied (sex, age, smoking habit, dyslipidemia, diabetes mellitus, prior percutaneous coronary intervention and prior surgery), the differences were of little relevance and were not statistically significant.
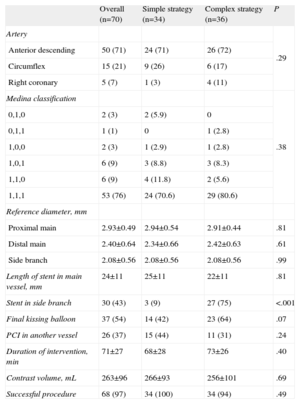
Procedural CharacteristicsProcedural characteristics are shown in Table 3. The majority of the bifurcation lesions in the 2 groups involved the left anterior descending coronary artery and its first diagonal branch. Most lesions were true bifurcations (85.6%); no differences in lesion classification were found between the two groups either in the intention to treat or the per protocol analysis.
Procedural Features in All the Lesions, Distributed According to Strategy in the Intention to Treat Analysis
| Overall (n=70) | Simple strategy (n=34) | Complex strategy (n=36) | P | |
| Artery | .29 | |||
| Anterior descending | 50 (71) | 24 (71) | 26 (72) | |
| Circumflex | 15 (21) | 9 (26) | 6 (17) | |
| Right coronary | 5 (7) | 1 (3) | 4 (11) | |
| Medina classification | .38 | |||
| 0,1,0 | 2 (3) | 2 (5.9) | 0 | |
| 0,1,1 | 1 (1) | 0 | 1 (2.8) | |
| 1,0,0 | 2 (3) | 1 (2.9) | 1 (2.8) | |
| 1,0,1 | 6 (9) | 3 (8.8) | 3 (8.3) | |
| 1,1,0 | 6 (9) | 4 (11.8) | 2 (5.6) | |
| 1,1,1 | 53 (76) | 24 (70.6) | 29 (80.6) | |
| Reference diameter, mm | ||||
| Proximal main | 2.93±0.49 | 2.94±0.54 | 2.91±0.44 | .81 |
| Distal main | 2.40±0.64 | 2.34±0.66 | 2.42±0.63 | .61 |
| Side branch | 2.08±0.56 | 2.08±0.56 | 2.08±0.56 | .99 |
| Length of stent in main vessel, mm | 24±11 | 25±11 | 22±11 | .81 |
| Stent in side branch | 30 (43) | 3 (9) | 27 (75) | <.001 |
| Final kissing balloon | 37 (54) | 14 (42) | 23 (64) | .07 |
| PCI in another vessel | 26 (37) | 15 (44) | 11 (31) | .24 |
| Duration of intervention, min | 71±27 | 68±28 | 73±26 | .40 |
| Contrast volume, mL | 263±96 | 266±93 | 256±101 | .69 |
| Successful procedure | 68 (97) | 34 (100) | 34 (94) | .49 |
PCI, percutaneous coronary intervention.
Data are expressed as no. (%) or mean±standard deviation.
In both analyses, final kissing balloon dilatation was more frequently employed in the complex strategy; this difference was not statistically significant in the intention to treat analysis (42% vs 64%), but were statistically significant in the per protocol analysis (33% vs 80%; P<.001).
There were no differences between the groups in procedure time or the amount of contrast material employed in either the intention to treat or per protocol analysis.
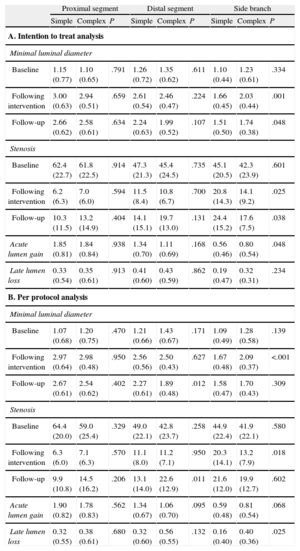
Angiographic ResultsThe angiographic results of the study are shown in Tables 4A and 4B (intention to treat analysis and per protocol analysis, respectively).
Angiographic Results
| Proximal segment | Distal segment | Side branch | |||||||
| Simple | Complex | P | Simple | Complex | P | Simple | Complex | P | |
| A. Intention to treat analysis | |||||||||
| Minimal luminal diameter | |||||||||
| Baseline | 1.15 (0.77) | 1.10 (0.65) | .791 | 1.26 (0.72) | 1.35 (0.62) | .611 | 1.10 (0.44) | 1.23 (0.61) | .334 |
| Following intervention | 3.00 (0.63) | 2.94 (0.51) | .659 | 2.61 (0.54) | 2.46 (0.47) | .224 | 1.66 (0.45) | 2.03 (0.44) | .001 |
| Follow-up | 2.66 (0.62) | 2.58 (0.61) | .634 | 2.24 (0.63) | 1.99 (0.52) | .107 | 1.51 (0.50) | 1.74 (0.38) | .048 |
| Stenosis | |||||||||
| Baseline | 62.4 (22.7) | 61.8 (22.5) | .914 | 47.3 (21.3) | 45.4 (24.5) | .735 | 45.1 (20.5) | 42.3 (23.9) | .601 |
| Following intervention | 6.2 (6.3) | 7.0 (6.0) | .594 | 11.5 (8.4) | 10.8 (6.7) | .700 | 20.8 (14.3) | 14.1 (9.2) | .025 |
| Follow-up | 10.3 (11.5) | 13.2 (14.9) | .404 | 14.1 (15.1) | 19.7 (13.0) | .131 | 24.4 (15.2) | 17.6 (7.5) | .038 |
| Acute lumen gain | 1.85 (0.81) | 1.84 (0.84) | .938 | 1.34 (0.70) | 1.11 (0.69) | .168 | 0.56 (0.46) | 0.80 (0.54) | .048 |
| Late lumen loss | 0.33 (0.54) | 0.35 (0.61) | .913 | 0.41 (0.60) | 0.43 (0.59) | .862 | 0.19 (0.47) | 0.32 (0.31) | .234 |
| B. Per protocol analysis | |||||||||
| Minimal luminal diameter | |||||||||
| Baseline | 1.07 (0.68) | 1.20 (0.75) | .470 | 1.21 (0.66) | 1.43 (0.67) | .171 | 1.09 (0.49) | 1.28 (0.58) | .139 |
| Following intervention | 2.97 (0.64) | 2.98 (0.48) | .950 | 2.56 (0.56) | 2.50 (0.43) | .627 | 1.67 (0.48) | 2.09 (0.37) | <.001 |
| Follow-up | 2.67 (0.61) | 2.54 (0.62) | .402 | 2.27 (0.61) | 1.89 (0.48) | .012 | 1.58 (0.47) | 1.70 (0.43) | .309 |
| Stenosis | |||||||||
| Baseline | 64.4 (20.0) | 59.0 (25.4) | .329 | 49.0 (22.1) | 42.8 (23.7) | .258 | 44.9 (22.4) | 41.9 (22.1) | .580 |
| Following intervention | 6.3 (6.0) | 7.1 (6.3) | .570 | 11.1 (8.0) | 11.2 (7.1) | .950 | 20.3 (14.1) | 13.2 (7.9) | .018 |
| Follow-up | 9.9 (10.8) | 14.5 (16.2) | .206 | 13.1 (14.0) | 22.6 (12.9) | .011 | 21.6 (12.0) | 19.9 (12.7) | .602 |
| Acute lumen gain | 1.90 (0.82) | 1.78 (0.83) | .562 | 1.34 (0.67) | 1.06 (0.70) | .095 | 0.59 (0.48) | 0.81 (0.54) | .068 |
| Late lumen loss | 0.32 (0.55) | 0.38 (0.61) | .680 | 0.32 (0.60) | 0.56 (0.55) | .132 | 0.16 (0.40) | 0.40 (0.36) | .025 |
Data are expressed as mean (standard deviation).
The baseline angiographic characteristics of the 2 strategy groups did not differ in any of the 3 segments analyzed: the proximal and distal main branch and the side branch.
The type of revascularization strategy resulted in angiographic differences both in the side branch and, to a lesser extent, in the main vessel. In the side branch, the complex strategy was associated with less stenosis following revascularization and, consequently, with a significantly greater acute lumen gain. In the follow-up at 9 months, residual stenosis in the side branch was lower in the complex strategy group in the intention to treat analysis but not in the per protocol analysis, which showed significantly greater late lumen loss with the complex strategy. As a result, there were no differences in residual stenosis between the 2 treatment modalities.
Differences in the main vessel were observed only in the distal segment: following revascularization, the acute lumen gain was lower in the complex strategy group but this difference was not statistically significant; 9 months later, the distal segments in the complex strategy group showed greater residual stenosis (significantly greater in the per protocol analysis) than those in the simple strategy group. These angiographic findings were similar to those observed among the 53 lesions classified as Medina 1,1,1.
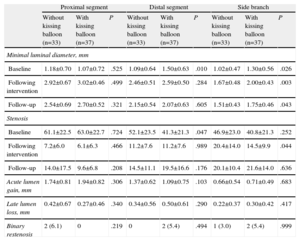
Table 5 shows the influence of final kissing balloon inflation on the angiographic results. The patients who underwent this technique tended to present better results in the proximal segment of the main vessel and poorer results in the distal segment with respect to acute lumen gain and late lumen loss. A possible explanation for these findings is that restenosis of the proximal segment developed only in those lesions in which the kissing balloon was not employed (6.1% vs 0%), whereas the opposite occurred with restenosis of the distal segment (0% vs 5.4%). In the side branch, the use of the kissing balloon had no apparent influence on the angiographic results, except for a significantly lower rate of stenosis following the procedure, an effect that disappeared during follow-up.
Angiographic Results With and Without Postdilatation Using Kissing Balloon
| Proximal segment | Distal segment | Side branch | |||||||
| Without kissing balloon (n=33) | With kissing balloon (n=37) | P | Without kissing balloon (n=33) | With kissing balloon (n=37) | P | Without kissing balloon (n=33) | With kissing balloon (n=37) | P | |
| Minimal luminal diameter, mm | |||||||||
| Baseline | 1.18±0.70 | 1.07±0.72 | .525 | 1.09±0.64 | 1.50±0.63 | .010 | 1.02±0.47 | 1.30±0.56 | .026 |
| Following intervention | 2.92±0.67 | 3.02±0.46 | .499 | 2.46±0.51 | 2.59±0.50 | .284 | 1.67±0.48 | 2.00±0.43 | .003 |
| Follow-up | 2.54±0.69 | 2.70±0.52 | .321 | 2.15±0.54 | 2.07±0.63 | .605 | 1.51±0.43 | 1.75±0.46 | .043 |
| Stenosis | |||||||||
| Baseline | 61.1±22.5 | 63.0±22.7 | .724 | 52.1±23.5 | 41.3±21.3 | .047 | 46.9±23.0 | 40.8±21.3 | .252 |
| Following intervention | 7.2±6.0 | 6.1±6.3 | .466 | 11.2±7.6 | 11.2±7.6 | .989 | 20.4±14.0 | 14.5±9.9 | .044 |
| Follow-up | 14.0±17.5 | 9.6±6.8 | .208 | 14.5±11.1 | 19.5±16.6 | .176 | 20.1±10.4 | 21.6±14.0 | .636 |
| Acute lumen gain, mm | 1.74±0.81 | 1.94±0.82 | .306 | 1.37±0.62 | 1.09±0.75 | .103 | 0.66±0.54 | 0.71±0.49 | .683 |
| Late lumen loss, mm | 0.42±0.67 | 0.27±0.46 | .340 | 0.34±0.56 | 0.50±0.61 | .290 | 0.22±0.37 | 0.30±0.42 | .417 |
| Binary restenosis | 2 (6.1) | 0 | .219 | 0 | 2 (5.4) | .494 | 1 (3.0) | 2 (5.4) | .999 |
The data are expressed as no. (%) or mean±standard deviation.
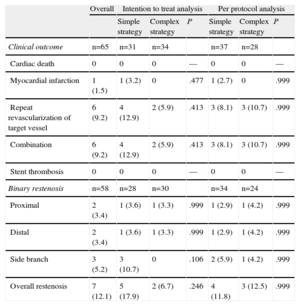
In our study, the overall rate of binary restenosis was 12.1% and the rate in the side branch was 5.2%. The intention to treat analysis revealed a nonsignificant trend toward a lower incidence of overall restenosis with the complex strategy (17.9% vs 6.7%). However, due to the results in the side branch (10.7% vs 0%; P=.10), these findings were not observed in the per protocol analysis, in which both strategies were associated with similar rates of restenosis, both overall (11.8% vs 12.5%) and in the side branch (5.9% vs 4.2%) (Table 6).
Clinical and Angiographic Follow-up (Binary Restenosis) at 9 Months
| Overall | Intention to treat analysis | Per protocol analysis | |||||
| Simple strategy | Complex strategy | P | Simple strategy | Complex strategy | P | ||
| Clinical outcome | n=65 | n=31 | n=34 | n=37 | n=28 | ||
| Cardiac death | 0 | 0 | 0 | — | 0 | 0 | — |
| Myocardial infarction | 1 (1.5) | 1 (3.2) | 0 | .477 | 1 (2.7) | 0 | .999 |
| Repeat revascularization of target vessel | 6 (9.2) | 4 (12.9) | 2 (5.9) | .413 | 3 (8.1) | 3 (10.7) | .999 |
| Combination | 6 (9.2) | 4 (12.9) | 2 (5.9) | .413 | 3 (8.1) | 3 (10.7) | .999 |
| Stent thrombosis | 0 | 0 | 0 | — | 0 | 0 | — |
| Binary restenosis | n=58 | n=28 | n=30 | n=34 | n=24 | ||
| Proximal | 2 (3.4) | 1 (3.6) | 1 (3.3) | .999 | 1 (2.9) | 1 (4.2) | .999 |
| Distal | 2 (3.4) | 1 (3.6) | 1 (3.3) | .999 | 1 (2.9) | 1 (4.2) | .999 |
| Side branch | 3 (5.2) | 3 (10.7) | 0 | .106 | 2 (5.9) | 1 (4.2) | .999 |
| Overall restenosis | 7 (12.1) | 5 (17.9) | 2 (6.7) | .246 | 4 (11.8) | 3 (12.5) | .999 |
Data are expressed as no. (%).
The overall rate of adverse cardiac events was 9.2%, and there were no differences between strategies in either of the 2 analyses (Table 6). There were no cases of cardiac death (of the 3 deaths, 1 was due to an occupational accident, another to trauma, and the third to sepsis). There was only 1 case of acute coronary syndrome without ST segment elevation, occurring in the simple strategy group, which required repeat revascularization of the target vessel due to severe restenosis prior to stenting. There were no cases of stent thrombosis during follow-up.
DISCUSSIONIn this study, there were no significant or relevant differences in the revascularization of bifurcation lesions with EES between the simple and the complex approach with T-stenting.
Bifurcation lesions constitute a common target of coronary interventional procedures. However, these lesions continue to be associated with poorer clinical and angiographic results12; therefore, it is important that the dilemma concerning the best strategy for their percutaneous revascularization be resolved.
The purpose of the so-called complex techniques, those involving deliberate implantation of stents in the main vessel and side branch, is to scaffold the entire anatomy of the bifurcation (including the carina) with metal, with the least possible distortion. There is no perfect complex technique because the degree of coverage of the side branch ostium weighs against the distortion of the bifurcation anatomy by the superimposition of different stent layers.13 T-stenting using the T and small protrusion technique (implantation of the side branch stent with a slight protrusion into the main vessel) is highly attractive since it combines optimal side branch coverage, little anatomical distortion throughout the entire bifurcation, and technical simplicity.
Although the simple strategy has been compared with the complex strategy in bifurcated lesions with DES implantation,2–7 until now there have been no comparisons of strategies involving second-generation DES. Among these, the EES has shown an optimal safety and efficacy profile in unselected populations.14 In addition, this strategy also combines a series of features that make it attractive in the revascularization of bifurcation lesions: first, it has demonstrated efficacy in avoiding restenosis in small vessels15 and side branch vessels are usually small; second, the design of the cobalt-chromium platform, with its thin structure and large cells, has been shown to be highly beneficial for the preservation of the side branch16; it also enables adequate expansion in the struts opposite the side branch ostium.17 Moreover, it has been reported to have an optimal safety profile in the treatment of bifurcation lesions, even superior to that of other second-generation DES.8,18
Comparison Between the Strategies According to Their Angiographic ResultsIn this study population, the approach to bifurcation lesions using EES was associated with an overall rate of binary restenosis at 9 months of 12.1% and of restenosis in the side branch of 5.2%.
Our study is the first to compare simple and complex T-stenting strategies using a second-generation DES. The complex approach tended to result in a lower rate of binary restenosis, due to the better outcome in the side branch. However, given the crossover rate of 17.1%, we considered it important to review that finding according to an analysis of the treatments actually carried out; in the per protocol analysis, we found no differences between the 2 strategies in terms of the overall rate of binary restenosis and the rate of side branch restenosis.
Despite this lack of a difference in the primary endpoint, the revascularization strategy was a determinant of the differential angiographic outcome both in the side branch and in the main vessel. In the side branch, although the complex strategy initially results in a greater lumen gain, subsequently, during follow-up, there is greater late lumen loss; thus, the final outcome of the 2 strategies is comparable.
In the main vessel, and specifically in the distal segment, the impact also differs depending on the strategy: with the complex strategy, due either to the implantation of a stent in the side branch or to the higher rate of final kissing balloon,19 the lumen gain following the procedure was less pronounced and there was greater residual stenosis on angiographic follow-up than with the simple strategy.
Clinical Outcome According to the Revascularization StrategyThe overall rate of adverse cardiac events in our study was 9.2%, similar to that reported in other trials of the use of EES in bifurcation lesions,17,20 and there were no differences between strategies. The most common adverse event was the need for repeat revascularization; in the majority of cases, repeat revascularization was not required because of ischemia, but was indicated by the operator performing angiographic follow-up. There were no cases of cardiac death or of definite or probable stent thrombosis.
LimitationsThe major limitation of this study is the sample size, which was smaller than the number established prior to commencement, due to low recruitment among the centers that initially agreed to participate; ultimately, the decision was made to recruit patients in a single center, and their inclusion was completed within a time period that we consider adequate. With the final sample size, the power of the statistical test, maintaining a level of significance of 95%, was 45%. Thus, the study is clearly limited in its capacity to demonstrate statistically significant differences in the results.
In addition to the insufficient sample size, there are other limitations, such as the loss of patients to follow-up and the crossover rate. With respect to the former, the angiographic follow-up was incomplete (84%) for various reasons: death, development of serious disease (cancer) that made angiographic follow-up unjustifiable, and the refusal of some patients who were asymptomatic. In addition, there was a high crossover rate, especially in the complex strategy group, usually based on the operator's discretion.
Randomization was not stratified for potential confounding factors; however, the procedures were performed in a single interventional cardiology unit and with identical operative criteria.
CONCLUSIONSIn general, EES were associated with low rates of angiographic binary restenosis and of adverse cardiac events in the treatment of bifurcation lesions, most of which were true bifurcation lesions. In this trial, the first performed with EES to define the best strategy in the treatment of bifurcation lesions, the complex strategy with T-stenting was not found to offer apparent clinical or angiographic advantages over the simple strategy. However, because of the low statistical power of our study, further studies are required to elucidate this issue.
FUNDINGThis study was partially funded by Abbott Vascular.
CONFLICTS OF INTERESTNone declared.
Study sponsored by the Fundación Española del Corazón.