Keywords
INTRODUCTION
Percutaneous coronary interventions (PCI) are now the most frequently used means for achieving coronary revascularization, and are a recognized alternative to surgery for nearly 95% of coronary lesions. Technological advances in the materials as well as improvements in adjunct pharmacological products have resulted in a more refined technique and reductions in related mortality and morbidity, with current estimates of 0.5%-1% mortality, 1%-2% acute myocardial infarction (AMI), and less than 0.5% urgent surgery. The majority of Spanish catheterization laboratories have reached these figures.1-3
The 2 main PCI-related complications are coronary occlusion and restenosis. Occlusion has been reduced with the use of stents, high-pressure stent implantation,4 and antiplatelet drugs (aspirin together with ticlopidine or clopidogrel) and glycoprotein IIb/IIIa receptor inhibitors, whereas restenosis continues to be the Achilles heel of interventional cardiology.5 The incidence of in-stent restenotic lesions is estimated at 10%-40%, depending on the characteristics of the patient and the lesion.2,5
Several strategies have been proposed to decrease or prevent this proliferative phenomenon, including new medical treatments, atherectomy, laser procedures, intracoronary brachytherapy,6 and recently antiproliferative drug-eluting stents.
The aim of this study was to assess the efficacy, effectiveness, and safety of stents coated with antiproliferative drugs for the treatment of coronary stenosis, and to perform an analysis in a hypothetical cohort of Spanish patients to determine the economic impact of using the new stents as compared to conventional uncoated stents.
MATERIALS AND METHODS
A systematic review was undertaken of the literature, with searches in MEDLINE, EMBASE, the Science Citation Index, and The Cochrane Library up to January 2004, and in several information sources, including registries of clinical trials, conference presentations, and Internet directories and search engines. The descriptors or free-text terms used (adapted to each database) were eluted stents, eluting stents, coated stents, stents, drug implants, drug delivery systems, rapamycin, sirolimus, paclitaxel, taxol, actinomycin, taxane, tranilast, trapidil, dexamethasone, batimastat, and dactinomycin.
Original studies, whether published or not and using any design, were retrieved. The inclusion criteria were as follows: studies on antiproliferative drug-coated stents performed in humans; studies assessing the outcome of treatment for coronary stenosis in terms of major adverse coronary events (MACE), or in terms of a combined outcome including death, AMI and the need for revascularization (coronary bypass surgery or PCI); and publication in English, French, Italian, or Spanish. In addition, a manual search was done of the literature references included in the articles retrieved.
The following data were compiled according to a specific protocol: type of publication, country, study design, sample size, participant characteristics, medical history, inclusion and exclusion criteria, comparison groups, characteristics of the intervention, follow-up and assessment, compliance and losses, statistical analysis, and endpoints. When several manuscripts included the same or a similar study population, the most complete data and results were used. Internal validity of the published studies was assessed independently by 2 appraisers, following the criteria proposed by the Evidence-Based Medicine Working Group.7
The direction of the effect was considered in the between-group comparison of MACE rates and, when the available data allowed it, categorical results were expressed as the relative risk (RR) or the number of persons who needed to be treated to prevent one adverse outcome (NNT).
In addition to the qualitative synthesis, a quantitative synthesis (meta-analysis) of endpoints evaluated in the same way was done in studies considered to be comparable and/or homogeneous. We also performed an analysis to detect the presence of statistical heterogeneity (Q statistic). The fixed-effects model (Mantel-Haenszel method) as well as the random-effects model (Dersimonian-Laird method) were both applied to calculate the summary RR and the 95% confidence interval (CI). The meta-analysis was conducted with the Meta-analyst© program developed by Joseph Lau of the Center for Health Services Research of the New England Medical Center.
To analyze the economic impact of using the new stents as compared to conventional stents, the market price, and the results from the previous efficacy/effectiveness review were used, and various information sources from our setting were consulted, mainly the Registro Español de Hemodinámica y Cardiología Intervencionista3 (Spanish Registry of Cardiac Catheterization and Interventional Cardiology) and a cost-effectiveness report on sirolimus-eluting stents sponsored by the manufacturer.8 When the available evidence was incomplete, experts in the field were contacted. The analysis was performed from the perspective of hospitals in Spain with a time horizon of one year, and the neutral price of the new stent was calculated as that which, according to standard practice and substituting the conventional stent, would not modify the overall estimated cost of the percutaneous coronary procedure.
RESULTS
Efficacy, Effectiveness, and Safety of the Antiproliferative Drug-Eluting Stent
Twelve published studies meeting the inclusion criteria were identified,9-20 some of them reported in more than 1 publication. Seven of these studies assessed sirolimus (rapamycin)-coated stents9-15 and 5 paclitaxel-coated stents.16-20 Seven had experimental designs (randomized, controlled clinical trials [RCTs]).10-12,16,17,19,20 The others were prospective clinical series without a control group9,13,14,18 and one series with a historical control group,15 assessing coronary lumen parameters (angiography and intravascular ultrasound) before and after the procedure and clinical aspects (MACE) only after the procedure. Evaluation of the methodological quality of the 12 studies identified showed that 7 of them used randomization and blinding, 6 performed an intent-to-treat analysis, 11 had adequate follow-up and control of the loss of subjects (<15%), 8 showed the between-group comparability at the beginning of the study, and 7 at the end of follow-up.
Ongoing (unpublished) trials with various antiproliferative drugs were also retrieved. In some only the preliminary results were available, whereas others had been halted due to the development of restenosis and significant adverse effects (ACTION trial with actinomycin-D, BRILLIANT, and BATMAN trials with batimastat, PRESENT I trial with tacrolimus, and SCORE trial with QuaDS-QP2).21 These latter studies were not included in this review.
Table 1 presents the main characteristics of the RCTs retrieved (published or ongoing), allowing evaluation of their homogeneity and comparability. Review of the published RCTs shows that patients treated with sirolimus (RAVEL10 trial and SIRIUS11,12 trials) or paclitaxel (TAXUS16,17,19 trials, ASPECT20 trial) for new lesions less than 30 mm long in vessels 2.5-3.5 mm in diameter presented better angiographic and intravascular sonographic outcome (minimal lumen diameter, stenosis diameter, late lumen loss and incidence of restenosis) than the groups treated with conventional stents (significant differences for most of these parameters at 6-9 months of follow-up). The incidence of MACE at 6-12 months was significantly lower in the group treated with coated stents, mainly because fewer revascularization procedures were required. The NNT to prevent revascularization with the new stents was less than 15 in all cases (Table 2). The thrombosis rate was 0%-1.1% with the drug-coated stent and 0%-0.8% with the conventional stent, with no statistical differences between the two stent types.
As seen in the review of observational studies, when coated stents were applied to treat patients with in-stent restenosis (ISR registries from Rotterdam13 and Brazil14 with sirolimus, and TAXUS III18 registry with paclitaxel), follow-up results at 4-12 months were poorer than those obtained in previous studies in patients with new lesions. The published preliminary results (30 days of follow-up) of the RESEARCH15 registry with sirolimus, involving patients with complex lesions and acute coronary syndrome, has shown success rates (MACE) and post-procedure complications similar to those of a historical cohort that received conventional stents. The FIM (First-in-Man) clinical series compared two different sirolimus-releasing formulations, with somewhat more favorable outcome at 2 years of follow-up for the group with the slow-release formulation.9,22-24
Several ongoing trials (ARTS II, BIFURCATION, DELIVER II, TAXUS V-VII, among others) have applied sirolimus-eluting or paclitaxel-eluting stents in more complex lesions and for in-stent restenosis, and some have studied other antiproliferative drugs, such as the PRESENT and EVIDENT trials with tacrolimus, and the FUTURE I-II trials with everolimus.
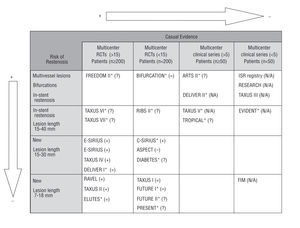
Figure 1 shows all the studies identified (published or unpublished), the direction of the effect found for MACEs according to the study design (experimental or not, number of participating centers and sample size), and the risk for developing restenosis among the patients included, defined by type of lesion (location, vessels affected and length). More than half the studies with a higher capability for demonstrating causal evidence (RCTs) are now ongoing and the majority include patients with a lower risk for restenosis (new lesions and shorter lesions).
Fig. 1. Studies identified (published and ongoing) according to the level causal evidence and the patients' risk of restenosis. + indicates positive effect, i.e., statistically significant clinical benefit (reduction in the rate of major adverse coronary events) with use of drug-eluting stent as compared to conventional stent; =, no effect, i.e., no significant differences between groups; , negative effect, i.e., statistically significant risk with use of drug-eluting stent as compared to conventional stent; ?, results still not available; N/A, not applicable, i.e., no randomized control group was used. *Ongoing study. The C-SIRIUS, BIFURCATION (in which the control group was angioplasty) and ELUTES studies were published during the peer review of this manuscript.
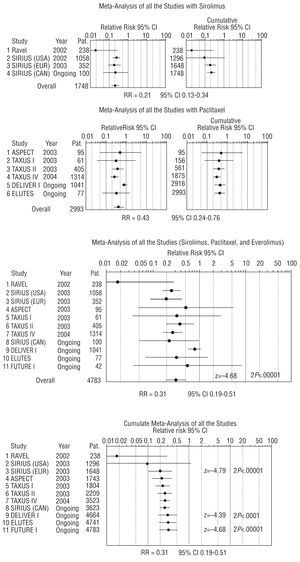
Meta-analysis of the RCTs was only done for revascularization rates (target lesion revascularization [TLR] or, when this data was not available, target vessel revascularization [TVR]), since it was the only clinical outcome among the MACEs that demonstrated significant differences between the comparison groups. Thirty-seven patients from the ASPECT trial who received a different antiplatelet treatment than patients in the remaining studies and who presented adverse effects were excluded from the meta-analysis. The results of the meta-analysis (Figure 2) indicate that the need for revascularization may be reduced by 69% (RR=0.31; 95% CI, 0.19-0.51). After studying possible causes of clinical heterogeneity, statistical heterogeneity was detected and a random-effects model was applied (P<.1; Q test). The analysis of subsets according to specific characteristics, such as the type of antiproliferative drug, type of lesion, duration of follow-up, type of revascularization assessed (TLR and/or TVR), or whether the study had been published or not, only demonstrated a somewhat more favorable outcome for sirolimus stents (RR=0.21; 95% CI, 0.13-0.34) than for paclitaxel stents (RR=0.43; 95% CI, 0.24-0.76). In addition, paclitaxel-eluting stents presented more variable outcome in the studies analyzed and their benefit in complex lesions was less conclusive.
Fig. 2. Association between the revascularization rate and treatment with antiproliferative drug-eluting stent: meta-analysis with random-effect model (Dersimonian-Laird method). Pat. indicates patients; RR, relative risk with the random-effects model; CI, confidence interval. The C-SIRIUS and ELUTES studies were published during the peer review of this manuscript.
To verify the robustness or stability of the final measure obtained, we performed a sensitivity analysis in order to determine the influence of each of the studies on the overall estimation of effect; there were no substantial changes in the results.
Cost Analysis
The aim of the cost analysis was to determine the economic implications of treating a cohort of patients with coronary stenosis (equal to the total number of patients treated during 2002 in Spain)3 by initial implantation of either a conventional stent or an antiproliferative drug-eluting stent. Table 3 shows the total cost at one year for 29 640 patients assuming average effects (not extreme rates of restenosis and revascularization) and according to the scientific evidence reviewed and the available cost data. Although use of the new stent leads to reductions in stenosis after the first procedure and consequently, the need for revascularization, their generalized use at current prices would imply an overall increase in funding. For every 1000 new patients, generalized use of coated stents instead of conventional stents would involve an additional cost of €818 718, that is, €819 per patient.
The neutral price of the new stent, that is, the value required for the new stent to avoid increasing the overall cost estimate of the conventional stent would be €1448, which is €552 less than the cost of the sirolimus-eluting stent used in the calculation (€2000 in 2004, approximately twice that of the conventional stent). Since the market price of the conventional stent may vary, the following formula was used to determine the neutral price of the coated stent based on the price of the conventional stent:
Neutral price of the coated stent =(1935.201+[price of conventional stentx4427])/4394
Assuming effect rates that minimize and maximize the total annual costs (sensitivity analysis according to the restenosis avoided), use of the new stent would imply an additional cost of €879 and €396 per patient, respectively. Estimated neutral cost for these 2 scenarios would range from €1407 to €1733, respectively.
DISCUSSION
Antiproliferative drug-eluting stents have generated high interest and expectations in the field of interventional cardiology. Nonetheless, published studies with the most robust design (RCTs) investigating the efficacy, effectiveness and safety of sirolimus or paclitaxel-coated stents have been limited to a highly selected population, with a low or moderate risk for restenosis.
Meta-analysis of the RCTs identified (published or unpublished) showed that the need for revascularization could be reduced by 49% to 81% when drug-eluting stents are used to treat new lesions and relatively non-complex lesions. Evidence from studies other than RCTs and ongoing studies in more complex lesions and/or in patients at a higher risk for restenosis is less promising in terms of absolute frequency. The results are generally better, however, than when conventional stents are used, and the decrease in relative risk seems to be similar in magnitude.
The concept or definition of restenosis and the preoccupation with the study of the coronary lumen have been points of conflict among interventional cardiologists for many years.25,26 The problems derived from performing follow-up angiographies and from interobserver and intraobserver variability, in addition to the poor angiographic and clinical correlation, have led to the use of clinical results (MACE) as indicators of restenosis. When a combination of different variables is used, a smaller sample size is needed to obtain significant differences between the groups compared; however, along with the increased precision obtained, this approach may generate confusion as to the true effect.27 In general, the studies reviewed showed significant differences in only one of the outcome variables: the need for revascularization. Although clinically relevant, the need for revascularization is still an intermediate outcome (not an endpoint) depending primarily on medical criteria, and it does not incorporate the impact on the patient's perception of health in a standardized manner.
With regard to adverse events, a higher frequency of incomplete apposition has been reported in the group receiving drug-coated stents. However, 12-month follow-up showed no increase in late thrombosis or MACEs in these patients.8,29 In addition, coated stents (Cypher® stents) have been related with more frequent development of subacute thrombosis and hypersensitivity reactions. In November 2003 the U.S. Food and Drug Administration (www.fda.gov/cdrh/safety/cypher.html) ratified the safety and efficacy of these devices when used under the conditions approved in April 2003: precise selection of stent size, appropriate selection of the patients (patients with new lesions ≤30 mm long occurring in 2.5- to 3.5-mm vessels), proper use of antiplatelet treatment (at least 3 months postimplantation) and use of adequate techniques for stent expansion.
Long-term outcome with the new stents is unknown. The longest follow-up period in a published clinical series is two years,24 and no new clinical events were observed. The resolution of other questions is still pending, for instance, whether or not the drug permanently inhibits neointimal growth or simply delays its formation, knowledge of the effect and safety of the polymers used, determination of the best antiproliferative agent and the role of the locally released drug dose, establishment of the efficacy of the new stents in different lesions than those studied up to now and in more unfavorable anatomic configurations, and finally, identification of patient subgroups in whom outcome with the new stents could be more relevant and cost-effective. Analyses in subsets of patients at a higher risk for restenosis (patients with diabetes, lesion in a narrow vessel and lesion located in the anterior descending artery) performed in one of the studies reviewed11 show higher clinical efficacy in these groups. These results should be confirmed in studies specifically designed for this purpose.30
At the market price, the generalized use of coated stents instead of conventional stents with a one-year time horizon would imply higher overall expenditure in all cases from the hospital's perspective. In this scenario, variations in stent price would change their economic impact. When viewed relative to the total cost per patient, the added expenditure does not seem so important, since revascularization surgery itself costs more than €6000 per intervention. Nonetheless, we still do not know how these stents will be used in actual practice. We assumed similar practice in the 2 cohorts of patients. However, it is possible that the indications for the new stents will be extended and their use generalized, as has occurred with other advances in medical technology.1
This study is not devoid of limitations. There can be selection bias in systematic reviews, as a result of inappropriate literature searching or of the so-called publication bias (studies in which results are negative tend to have a lower probability of being published than those with positive results),31 and this would lead to overestimation of the observed effect. Our inclusion of unpublished studies and searches in several information sources has probably reduced this possibility. Published studies that had been halted because of the development of adverse events with use of the new stents were not included. These studies, conducted with other antiproliferative drugs, had been halted, manufacture of the stents discontinued and related research stopped; thus, they are not likely to have influenced the effectiveness and safety results of the drug-eluting stents assessed. Another limitation is the fact that the cost analysis is simplified and approximate; it is not a study of cost-effectiveness. It was assumed that the other possible outcomes of angioplasty with stent implantation (success, AMI, death, and adverse effects) would be similar with either conventional or drug-coated stents, and that the use of standard balloons, conventional stents, bypass grafts or other devices (cutting balloons, atherectomy, etc) would also be similar when the need for revascularization was produced. The estimated percentage of revascularization procedures used can vary between hospitals and may change in the future with the increasing use of drug-eluting stents, but for the moment those presented are closest to current practice. Moreover, we applied the data on the cost-effectiveness of the sirolimus-eluting stent from a prior study8 that consulted the Soikos database on health care costs (2002). This not a free-access information source and does not clearly identify the basis for all the values provided. In addition it is unknown whether other direct costs (e.g. hours of nursing care, number of medical visits, postprocedure rehabilitation, etc) were taken into account.
CONCLUSIONS
The results of this study indicate that in comparison with conventional stents, treatment for coronary artery stenosis with sirolimus- or paclitaxel-eluting stents can lower the need for revascularization due to clinical restenosis up to 69% in single, new lesions under 30 mm in length, in vessels 2.5-3.5 mm in diameter at 12 months' of follow up. No other clinical benefits were demonstrated.
From the perspective of the hospital and within a time horizon of one year, generalized use of drug-coated stents at market prices would imply higher overall expenditure in all cases.
Although there are several reasons for optimism with the development of antiproliferative drug-eluting stents, more randomized controlled studies are needed to determine the type of patients and lesions likely to obtain the greatest benefits, thereby contributing to more cost-effective use of this technology.
Full English text available at: www.revespcardiol.org
See editorial on pages 608-12
ABBREVIATIONS
RCT: randomized clinical trial.
MACE: major adverse coronary events.
AMI: acute myocardial infarction.
PCI: percutaneous coronary intervention.
NNT: number needed to treat.
RR: relative risk.
This study is an extension and update of the report, "Stents recubiertos de fármacos antiproliferativos para el tratamiento de la estenosis coronaria" (Antiproliferative drug-coated stents for the treatment of coronary artery stenosis), sponsored by the Agència d´Avaluació de Tecnologia i Recerca Mèdiques (Catalan Agency for Health Technology Assessment and Research) for the Ministerio de Sanidad y Consumo (Ministry of Health and Consumer Affairs), delivery date December 2002.
Correspondence: Dra. G. Oliva.
Área de Evaluación en Servicios Sanitarios. Agència d'Avaluació de Tecnologia i Recerca Mèdiques.
Esteve Terradas, 30. Recinte Parc Sanitari Pere Virgili.
Edifici Mestral, 1.a planta. 08023 Barcelona. España.
E-mail: goliva@aatrm.catsalut.net