Early reperfusion of an occluded coronary artery is the primary goal of acute myocardial infarction (AMI) therapy to interrupt the “wave front” of myocardial necrosis and to promote the onset of the healing process. However, the restoration of blood flow itself is often associated with ischemia-reperfusion injury in the infarcted area, resulting in a sizable proportion of patients experiencing microvascular obstruction (MVO).1 This phenomenon, related to a worse prognosis and left ventricular remodeling, is the consequence of both functional and morphological changes in the myocardial microvasculature. A huge disruption of the microvessel network has been widely described and observed in experimental models.1,2 Interestingly, several studies have shown that MVO may evolve over time, with its dynamic time course starting in the hospital phase and lasting for weeks after AMI.3–5 During this process, angiogenesis is of the utmost importance both for its potential to salvage ischemic myocardium, providing oxygen and nutrients at early stages after AMI, and to prevent the transition to heart failure.6 Neoangiogenesis, or simply angiogenesis, represents the emergence of newly formed microvessels from pre-existing capillaries in response to multiple signals such as hypoxia, growth factors, and nitric oxide. It is an extremely regulated process requiring interactions among endothelial cells, extracellular matrix and surrounding cells and is mediated by growth factors, their receptors, and intracellular signaling.7 The most widely investigated angiogenetic factors in the setting of AMI are hypoxia-inducible factors (HIFs), vascular endothelial growth factor (VEGF) and angiopoietins. HIFs are transcription factors that respond to decreases in available oxygen in the cellular environment or hypoxia. In an animal model of mice constitutively expressing HIF–1-alpha, cardiac function after AMI improved, associated with increased VEGF expression and angiogenesis.8 HIF–2-alpha also induces expression of various angiogenic genes, such as VEGF or angiopoietins9; in particular, Skuli et al.10 suggest that HIF-alpha has complementary properties to HIF–1-alpha: its absence in endothelial cells provokes failure of maturation of the newly formed vessels.10.1016/j.rec.2017.06.019
Members of the VEGF family play a predominant role in the coronary system; VEGF acts by binding to its receptor, VEGF receptor 2 (VEGFR2), promoting endothelial cell survival, proliferation, and migration.11 Another member of the VEGF family of growth factors that preferentially binds to VEGF receptor 1 (VEGFR1), placental growth factor, also activates angiogenesis in ischemic tissues through amplification of VEGF-dependent signaling and promotion of the recruitment of proangiogenic VEGFR1, expressing myelomonocytic cells. In addition, angiopoietins are involved in the setting of AMI; in particular, Galaup et al.12 reported, in angiopoietin-like-4-deficient mice, increased vascular permeability, hemorrhage, edema, inflammation, infarct extension, and MVO incidence, suggesting that angiopoietin-like-4 might constitute a relevant target for therapeutic vasculoprotection. A significant reduction in angiopoietin-2 (Ang-2) levels and its receptor (Tie-2) was observed by Lee et al.13 in patients with AMI 48hours after symptom onset, with a subsequent increase in Ang-2 levels after a few weeks.
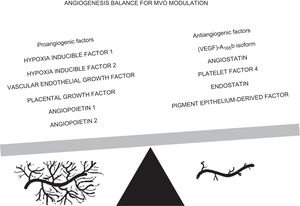
The study by Ríos-Navarro et al.,14 recently published in Revista Española de Cardiología, aimed to evaluate the neoangiogenetic potential of coronary serum obtained after AMI and the consequent MVO repair using a highly controlled swine model of AMI and MVO and an in vitro assay of coronary endothelial cell tubulogenesis, a previously validated model.2 The study confirms the dynamic pattern of MVO, with enhancement of infarct size if there is reperfusion and MVO recovery after a month. It is also widely established and reported in clinical studies that MVO occurs and reverses, with wide interpatient variability.4,5 Moreover, Ríos-Navarro et al.14 report that, in comparison with controls, coronary serum derived from AMI experiments exerted a potent neoangiogenic effect, with a peak during ischemia and immediately after reperfusion, with detectable effects during the first month after AMI. On the basis of these findings, they provide 2 main suggestions: a) molecular mechanisms able to restore the microvascular damage are already present before reperfusion; and b) the neoangiogenic effect exerted by coronary serum significantly contributes to the spontaneous resolution of MVO 1 month after reperfusion. However, neoangiogenesis is one of the mechanisms involved in MVO progression; systemic and local inflammation also plays a crucial role. Among previous studies, we reported that negative variation in inflammatory biomarkers are associated with MVO reversibility during the first week after AMI and that the absolute values of these biomarkers are higher in patients experiencing sustained MVO.15 In the same study, we also detected a significant rise in Ang-2 levels among patients with reversible MVO, in agreement with the experiments by Ríos-Navarro et al.14 With the aim of identifying the molecular mechanisms of microvessels restored by neoangiogenesis, the latter assessed circulating levels of HIF–1-alpha and showed that its time course peaked during ischemia and soon after reperfusion, mirroring the angiogenic effect of coronary serum on in vitro coronary endothelial cells; moreover, they were able to confirm this finding, observing a reduction of new capillary growth after neutralization of HIF–1-alpha with antibodies. They also assessed HIF–1-alpha messenger RNA expression at different time points and found high levels 1 week and 1 month after reperfusion, with a certain delay after AMI; however, angiogenesis was already promoted by HIF–1-alpha on coronary endothelium even before reperfusion; this implies a constitutive expression in the HIF–1-alpha gene. Similarly, levels of (VEGF)-A165b, an antiangiogenic factor that modulates vascularization, increased after ischemia onset, with a positive correlation with infarct size and a negative correlation with left ventricular ejection fraction.16 Thus, neoangiogenesis and, consequently, the restoration of microvessel integrity after AMI are the result of a balance between proangiogenic and antiangiogenic stimuli, as shown in the Figure; modulation of these molecular patterns with therapeutic interventions would be a strategic option to maximize neovasculature formation, microvascular density recovery, and microvascular perfusion. From this point of view, the study by Ríos-Navarro et al.14 may indicate the HIF–1-alpha pathway as one of the main therapeutic targets to intensify the spontaneous phenomenon of angiogenesis.
Given all these factors, the study is of particular interest; up to now, many studies have focused on angiogenesis in the setting of AMI, while few have investigated the role of angiogenesis and its mechanisms in MVO recovery. Many preclinical studies have tested proangiogenic therapies with discordant results,17 but they provided the basis for numerous clinical trials, leading to suggestions for the optimization of proangiogenic therapies, including stimulation of angiogenesis and, eventually, the use of progenitor cells. MVO progression contributes to left ventricular remodeling and prognosis; thus, future studies should target therapies that favorably affect MVO reversibility, with neoangiogenesis as a main therapeutic target.
CONFLICTS OF INTERESTNone declared.
.