Because of diagnostic and therapeutic advances in recent decades, a larger number of patients with congenital heart disease (CHD) are now reaching adulthood. Indeed, in North America, and possibly also in Spain, the adult population with complex CHD already exceeds the pediatric population.1 This improved life expectancy has unfortunately increased the incidence of complications that may not be amenable to surgical repair and/or are refractory to medical therapy,2–4 significantly increasing the risk of heart failure (HF). This risk is almost 50% for patients with Fontan circulation and 25% to 35% for those with a systemic right ventricle.
One of the major challenges posed by adult patients with CHD lies in the diagnosis of HF. By definition, these patients have an abnormal cardiac anatomy from birth and, although they have classic symptoms of HF, they sometimes show signs of HF (eg, low maximal oxygen uptake, elevated brain natriuretic peptide, and protein-losing enteropathy) without symptoms, making it very difficult to establish a universal definition of HF for this population. In addition, the management of HF in this group of patients poses a considerable challenge to cardiologists not only because of their heterogeneity, but also because of the different forms of presentation, which depend both on the underlying heart disease and on the type of repair. There is also the added difficulty caused by the absence of validated biomarkers that would permit monitoring of disease progression or established risk factors and allow estimation of prognosis and, obviously, the scant or even absent evidence that conventional HF treatment is effective in these patients.
Despite the major contribution of HF to the morbidity and mortality of adult patients with CHD, there are no sufficiently powered clinical trials to explain the role of medical therapy in this population. Although there are some randomized studies, most of the published data have been derived from small observational studies that included heterogeneous populations and had short follow-up times and low event rates. Accordingly, these studies do not allow definitive conclusions to be drawn about the efficacy of these drugs in this population. In patients with CHD with biventricular circulation and left ventricular dysfunction (systemic ventricle) as the main mechanism underlying the HF, it seems reasonable to apply the same therapy as that used for patients with acquired HF. However, these drugs can be harmful in some patient subgroups, such as people with single-ventricle physiology, mainly those converted to a Fontan circulation. In these patients, the use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers can lead to a reduced afterload that is not offset by an increased beat volume, further reducing cardiac output. Another important group comprises patients with transposition of the great vessels corrected with an arterial switch. Because these patients’ stroke volume significantly depends on heart rate, beta-blockers should be used with caution. Therefore, cardiologists must identify the pathophysiology of the underlying heart disease when choosing the most appropriate drug for each clinical situation. In addition, although the evidence is scarce,5 some patients with CHD could benefit from cardiac resynchronization therapy. However, resynchronization device implantation in these patients can be extremely complex or even unfeasible due to an “anomalous” position of the coronary sinus. Even when resynchronization is technically feasible, the existing evidence is insufficient to reliably identify those patients who would benefit from this therapy.
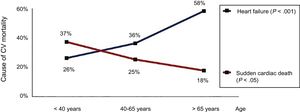
One indisputable fact is that in recent years the prevalence of HF has significantly increased in patients with CHD. A recent study estimated a first HF event incidence in adult patients with CHD of approximately 1.2/1000 patient-years, with a 5 times higher risk of death among those requiring hospitalization.6 In the last 5 years, HF hospitalization has exponentially increased among patients with CHD older than 40 years of age in the United Kingdom (unpublished data), which may explain why HF is now the leading cause of death in this group of patients3,7 (Figure 1). Due to this increase in HF prevalence, there has been a significant rise in the number of patients with CHD and refractory HF whose only therapeutic option is heart transplant. Although some studies indicate a slight uptick in the number of heart transplants in patients with CHD and biventricular circulation, the number of transplants in patients with single-ventricle circulation has not changed in recent years.8 According to data from the International Registry for Heart and Lung Transplantation,9 patients with CHD represent only 3% of the heart transplants performed worldwide. It is difficult to know exactly why the number of transplants in patients with CHD is significantly lower than in other patient populations. The absence of cardiologists and surgeons with experience of CHD in heart transplant units probably reduces the possible inclusion of these patients on the waiting lists. In addition, this group has high peritransplant mortality, with bleeding and primary graft failure being the most frequent causes of early mortality, making them less “attractive” candidates for heart transplant. Nonetheless, recent data indicate a significant increase in survival in these patients when cardiologists with experience in CHD are integrated into transplant groups and when the transplant is performed by a cardiac surgeon with experience in CHD.10 From these data, it can be concluded that close collaboration is needed between CHD and Heart Transplant Units to improve both transplant access and the prognosis of these patients.
Mortality from heart failure and sudden cardiac death in patients with congenital heart disease. Reproduced with permission from Oliver et al.7 CV, cardiovascular.
Crucially, the absence of specific transplant indication criteria in these patients puts this population at a clear disadvantage vs those with acquired heart disease when heart transplant candidates are being considered. Each case must be evaluated individually, particularly patients with complex CHD, and always with the involvement a cardiologist experienced in CHD. Once these patients have been placed on the heart transplant waiting list, it should be remembered that, although they have higher waiting list mortality2 and higher peritransplant morbidity and mortality, their posttransplant survival is higher than that of patients with acquired heart disease. In the last report of the International Society for Heart and Lung Transplantation,9 the average survival of patients with CHD who survived the first postheart transplant year was 15 years vs 10 years for patients with ischemic heart disease and 12 years for those with nonischemic heart disease. Recognized risk factors that increase heart transplant mortality in these patients are Fontan circulation, complex anatomy, multiple previous sternotomies, and pulmonary hypertension.
Factors that should be considered when the possibility of transplant or its indication is being assessed in these patients include the higher prevalence of HLA (human leukocyte antigen) antibodies, an altered cardiac anatomy due to previous interventions, the presence of anatomical anomalies such as transposition of the great vessels or dextrocardia, and multiple previous cardiac interventions that may increase the transplant risk. One of the most complex management decisions is when to refer a patient with CHD for heart transplant evaluation, particularly patients with Fontan circulation. As a general rule, the recommendation is that they be referred at an early stage, because many will develop multiorgan failure. These patients frequently show the presence of cardiorenal syndrome with intrinsic renal damage, even in the presence of normal creatinine values. Likewise, chronic elevation of venous pressure causes hepatic congestion and the subsequent development of liver fibrosis and/or cardiac cirrhosis.11 Therefore, when these patients are being evaluated for heart transplant, an exhaustive liver assessment should be performed, which should be carried out jointly by cardiologists with experience in CHD and hepatologists. Because both renal failure and advanced liver cirrhosis can be contraindications to heart transplant, these patients should be referred for evaluation before there is significant deterioration in these organs contraindicating isolated heart transplant. In addition, when decisions are being made regarding patient referral to a transplant unit, physicians must bear in mind that the criteria used for patients with acquired heart disease may not be applicable to this population. Patients with CHD experience a progressive deterioration in exercise capacity that leads to gradual changes in their physical activity, making assessment of their functional capacity extremely difficult. Transplantation should be part of the initial therapeutic arsenal and should be considered for patients with HF and progressive deterioration in functional capacity without other therapeutic options, although it is common for these patients to feel well and, therefore, fail to understand the benefit of a heart transplant.
One of the major advances in the treatment of advanced HF in patients with acquired heart disease is the development of ventricular assist devices (VADs), which are normally used as left ventricular support.12 Patients with CHD have a higher probability of having right HF, pulmonary hypertension, or residual shunts, making them less attractive candidates for these devices.13 Although the use of VAD in patients with acquired heart disease has exponentially increased in recent years, its use in patients with CHD remains infrequent. Recent data from the INTERMACS registry14 show that, of 16 182 patients who received a VAD in the United States between 2006 and 2015, only 126 (less than 1%) had CHD. Of these, 45 patients had a systemic right ventricle and 17 had a univentricular heart. In contrast to the situation for patients with acquired heart disease, who received a VAD predominantly as destination therapy, the most frequent indication for patients with CHD was as a bridge to transplant. In that study, a higher number of patients with CHD required biventricular assistance or a total artificial heart, which was associated with a higher risk of complications. The most interesting aspect of the study is that the survival of patients with CHD with biventricular circulation who received a VAD implantation in the systemic ventricle (including those with a systemic right ventricle) was the same as that of patients with acquired heart disease. These results are encouraging and open the door to the use of these devices in our patients, particularly as a bridge to heart transplant.
A group that can greatly benefit from advances in VADs is patients with a systemic right ventricle. The downsizing of these devices means that their implantation in a trabeculated ventricle is no longer a problem. Small case series have shown good short- and mid-term results, with the same complications as those in patients with acquired heart disease. In patients with single-ventricle physiology and Fontan circulation, VAD use is more complicated. Although surgical options have been proposed that allow the implantation of a Berlin Heart VAD (Berlin Heart AG, Berlin, Germany) in the Fontan circuit, the truth is that, if these patients require ventricular assistance, particularly those with preserved ventricular function, the only available option would be a total artificial heart (SynCardia Systems, Inc, Tucson, Arizona, United States), which, according to data from the INTERMACS registry, is associated with a higher risk of complications and higher mortality in these patients. In the case of patients with failure of the Fontan circulation, the ideal device would be a pump implanted in the Fontan circuit that increases pulmonary flow. Indeed, several studies of different devices are underway, and their use may be possible in our patients in the not-too-distant future.
A special population comprises patients with CHD who present with cardiogenic shock. These patients may benefit from support with extracorporeal membrane oxygenation (ECMO) or continuous flow pumps when oxygenation and ventilation are unaffected. In this situation, the extracorporeal circulation device should be implanted by someone with experience in CHD who perfectly understands the patient's anatomy and circulation, because standard cannulation may be contraindicated for some patients, particularly those with a single-ventricle physiology.
In conclusion, it is clear that HF is becoming more frequent in patients with CHD, not only in CHD clinics, but also in inpatient cardiology wards. The number of patients requiring a heart transplant and/or VAD is expected to rise exponentially in the next 10 years. The adequate selection of optimal candidates for heart transplant continues to be a challenge, but it is us, cardiologists specializing in adult CHD, who, in close collaboration with heart transplant units, must establish the transplant criteria in this population.
In this group of patients, ventricular function and/or functional class are probably not the only factors to be considered when decisions are being made regarding when to refer a patient for heart transplant evaluation, and more attention should be paid to the failure of other organs, such as the kidney or liver. For this reason, there is a need to promote the creation of multidisciplinary units specialized in the treatment of advanced HF in patients with CHD. These units should include at least 1 cardiologist with experience in CHD, a cardiologist with experience in heart transplant and VAD use, a hepatologist, and a cardiac surgeon with experience in CHD, in addition to psychological support, and this team should be coordinated by the CHD Unit.
CONFLICTS OF INTERESTNone declared.
.