Lysyl oxidase is overexpressed in the myocardium of patients with hypertensive cardiomyopathy. We aimed to explore whether patients with hypertensive-metabolic heart failure with preserved ejection fraction (HM-HFpEF) also have increased concentrations of circulating prolysyl oxidase (cpLOX) and its possible consequences.
MethodsWe quantified cpLOX concentrations in 85 nonischemic patients with stage C, HM-HFpEF, and compared them with those of 51 healthy controls. We also assessed the correlations of cpLOX with myocardial stiffness parameters, collagen turnover products and fibrogenic cytokines, as well as the predictive value of plasma proenzyme levels at 1-year of follow-up.
ResultsWe detected raised cpLOX values and found that they correlated with calculated E/E’ ratios and stiffness constants. The subgroup of patients with type I diastolic dysfunction showed a single negative correlation between cpLOX and B-type natriuretic peptide whereas patients with a restrictive diastolic pattern showed a strong correlation between cpLOX and galectin-3. Kaplan-Meier analysis revealed that cpLOX > 52.20 ng/mL slightly increased the risk of a fatal outcome (log-rank = 4.45; P = .034). When Cox regression was used, cpLOX was found to be a significant independent predictor of cardiovascular death or hospitalization due to the decompensation of HM-HFpEF (HR, 1.360; 95%CI, 1.126-1.638; P = .046).
ConclusionsPatients with symptomatic HM-HFpEF show high cpLOX serum levels associated with restrictive diastolic filling indices. These levels represent a moderate risk factor for poor clinical outcome. Throughout the natural history of HM-HFpEF, we observed that cpLOX concentrations were initially negatively correlated with B-type natriuretic peptide but positively correlated with galectin-3 as advanced diastolic dysfunction developed.
Keywords
Heart failure with preserved ejection fraction (HFpEF) is a complex clinical-hemodynamic syndrome of paramount importance surrounded by all kind of controversies, which are partly derived from its enormous heterogeneity.1,2 To address this issue, Shah et al.3,4 developed a phenotypic classification of this condition and categorized a common, garden-variety HFpEF (hereafter referred as hypertensive-metabolic HFpEF [HM-HFpEF]) as a distinct clinical-etiological entity that should be separated from other HFpEF forms, particularly ischemic and right sided HFpEF. Experimental work clearly supports the notion that nonscarring, interstitial, perivascular and predominantly subendocardial fibrosis by myocardial deposition of collagen types I and III is a key process accompanying HFpEF in nonischemic, hypertensive, and diabetic patients.5
Collagen fiber arrangement results from the action of lysyl oxidase (LOX), a copper-dependent extracellular matrix enzyme encoded by a gene located on the chromosome 5q23.3-31.2,6 which catalyzes the formation of highly reactive aldehydes from lysine residues in collagen and elastin precursors. This results in molecular cross-linking, which is essential for the stabilization of stiff collagen fibrils inside the myocardial matrix. A balanced deposit of this kind of fibrils plays a critical role in normal cardiac hemodynamics.7
Lysyl oxidase is synthesized in fibrogenic cells as a 50-kDa precursor111 proenzyme (pro-LOX), which is afterwards processed in an equimolar ratio to produce active LOX enzyme.8 Since LOX concentrations are almost negligible in the blood of healthy individuals, it has been suggested that LOX plasma might serve as a biomarker of organ fibrosis in several inflammatory and neoplastic disorders.9,10 Conversely, the identification of LOX as a possible plasma biomarker of rigid collagen deposition in heart disease has hardly been investigated, despite reports providing evidence that enzyme excess in cardiac tissue is associated with increased collagen I cross-linking and left ventricular (LV) stiffness in the failing heart of patients with hypertensive cardiomyopathy.11 To the best of our knowledge, no clinical studies have hitherto been reported to test blood LOX or pro-LOX in a “real-world” population of patients with HM-HFpEF.
METHODSStudy PopulationFrom April 2013 to December 2015, 205 patients treated on an ambulatory basis after hospital discharge with symptomatic severe HFpEF (New York Heart Association [NYHA] functional class II: 86 patients; NYHA functional class III-IV: 119 patients) of possible hypertensive and/or metabolic etiology were evaluated. Of note, only stable patients with antecedents of hypertension (presumably salt-dependent) and/or at least 1 other metabolic syndrome risk factor (ie, elevated fasting glucose, elevated waist circumference, elevated triglycerides or reduced high-density lipoprotein cholesterol)12 were screened for eligibility to enter the study. All patients had been hospitalized in the preceding 6 weeks due to an acute congestive decompensation, whether or not this had been accompanied by other cardiovascular disease (ie, acute coronary syndrome, uncontrolled brady- and tachyarrythmia, pericardial effusion with hemodynamic compromise, thromboembolic disease, or severe arterial hypertension) and/or noncardiovascular comorbidities. Diagnosis of noninvasive HFpEF was made according to Framingham criteria and the European Society of Cardiology recommendations (ie, all patients showing LV ejection fraction ≥ 50% and B-type natriuretic peptide (BNP) levels > 35 pg/mL).13 Endomyocardial biopsies or direct determination of LV filling pressures and relaxation time constants (τ) were not employed for diagnostic purposes in any of the patients. We systematically assessed patients for coronary artery disease and other non-HM cardiomyopathies through careful routine scrutiny of their clinical records, electrocardiograms, and heart ultrasound examinations. Stress testing (conventional treadmill exercise-electrocardiogram, dobutamine-atropine echocardiography with visual and/or standard 2-dimensional speckle tracking assessment, and 201Thallium-dipiridamol single-photon emission computed tomography scintigraphy) (18 procedures), coronary angiography (49 procedures) and/or delayed-enhancement cardiac magnetic resonance (24 procedures) was performed in 88 registered patients. Next, after applying all the exclusion criteria related to HFpEF phenotype (see below), the 132 remaining patients were categorized as having a definite HM-HFpEF syndrome. Finally, in accordance with our objectives, 47 out of 132 patients were excluded due to the coexistence of HM-HFpEF with some other potential extracardiac fibrotic disorders (see also below). In summary, 85 stable stage C HFpEF patients with a specific diagnosis of nonischemic hypertensive-metabolic phenotype, but without any other major fibrogenic diseases, were eligible to enter the study protocol. We included 51 age- and sex-matched healthy controls, 28 women and 23 men who were older than 60 years (mean 72 ± 4.5 years), had no significant diseases or major cardiovascular risk factors, and were not taking any medication. Participants who volunteered as controls were randomly selected from veteran hospital workers and patients’ relatives who met the above-mentioned criteria. The absence of pathological antecedents or significant cardiovascular risk factors was evaluated by history-taking, standard blood pressure determination, and routine cardiac ultrasound examination.
Exclusion CriteriaThe following exclusion criteria were related to the HFpEF phenotype (73 patients were excluded): a) a history of diagnosis of a well-documented acute coronary syndrome or unrecognized past acute myocardial infarction under the Minnesota code scheme (major Q/QS wave abnormalities)14 (41 patients); b) active ischemic heart disease evidenced by angina, positive stress testing, and/or abnormal cardiac magnetic resonance or angiographically proven significant coronary artery disease (15 patients); c) predominant right ventricular dilatation and/or dysfunction, cor pulmonale, severe tricuspid valve regurgitation or Doppler calculated pulmonary artery systolic pressure > 65mmHg (12 patients); d) serious valvular, pericardial or congenital heart disease, including hypertrophic cardiomyopathy (4 patients); and e) probable primary restrictive cardiomyopathy (senile cardiac amyloidosis) (1 patient).
The following exclusion criteria were related to possibly active fibroinflammatory conditions (47 patients were excluded): severe (Global Initiative for Chronic Obstructive Lung Disease grade III to IV) chronic obstructive lung disease and/or pulmonary fibrosis (14 patients), dementia with significant cognitive deterioration (11 patients), estimated glomerular filtration rate < 15mL/min/1.73 m2 (5 patients), hemoglobin < 9.0g/dL (4 patients), active collagenosis (2 patients), peripheral arterial disease with severe claudication (2 patients), chronic liver disease (2 patients), history of extensive therapeutic radiation (1 patient), clinically relevant hypo- and hyperthyroidism (1 patient), a history of solid organ transplantation (1 patient), a recent (< 3 months) cardiopulmonary resuscitation procedure (1 patient), serious trauma (1 patient), permanent stroke (1 patient), and major surgery (1 patient).
Study ObjectivesThe aims of this observational study in patients with HM-HFpEF syndrome were to examine: a) circulating prolysyl oxidase (cpLOX) concentrations; b) cpLOX correlations with myocardial stiffness indices; and c) plausible mechanisms of LOX humoral control; and d) cpLOX clinical predictive value.
Study VariablesThe study variables were as follows: a) for hemodynamic assessment: cpLOX, E/E’ ratio (as a surrogate for LV filling pressures) and estimated LV stiffness constant; b) for pathophysiological assessment: cpLOX (dependent variable): BNP and a selected panel of profibrotic cytokines and collagen turnover biomarkers (explicative variables) (see below); and c) for adjusted clinical outcome assessment: cpLOX and serum biomarkers with recognized HFpEF predictive value (BNP, galectin-3, soluble toll-like/interleukin-1 type-II receptor, growth differentiaton factor-15, matrix metalloproteinase (MMP) 2 and high-sensitivity troponin T).15
Laboratory AnalysisOnce patients were enrolled, blood samples were withdrawn from their left antecubital vein and were collected in uncoated serum and EDTA plasma tubes. The samples were allowed to coagulate at room temperature and were centrifuged at 3500g for 10minutes. Shortly after, sera and plasma were aliquoted and stored at −70°C until analysis. Quantifications of cpLOX (Uscn Life Science Inc; Ubei, China), galectin-3 (US Biological Life Sciences; Swampscott, Massachusetts, United States), transforming growth factor-ß1 (Boster Immunoleader; Fremont, California, United States), soluble toll-like/interleukin-1 type-II receptor (Swampscott), growth differentiaton factor-15 (Abnova Co; Taipei, Taiwan), carboxi-terminal propeptide of procollagen I (Uscn Life Science Inc), amino-terminal propeptide of procollagen III and carboxi-terminal telopeptide of collagen I (SunRed Biological Technologies; Shangai, China), MMPs (MMP-2 and MMP-9) and tissue inhibitor of MMP-1 (Boster Immunoleader) were performed in the stored sera by sandwich ELISA as stated in each manufacturer's specifications. Additionally, BNP and high-sensitivity troponin T were measured in fresh blood samples using highly sensitive and specific electrochemiluminescence immunoassays (Elecsys proBNP and Elecsys Troponin T hs, Roche Diag.; Manheim, Germany). The estimated glomerular filtration rate was calculated using de MDRD (Modification of Diet in Renal Disease) equation.
EchocardiographyTransthoracic echocardiography was twice repeated at the time of blood extraction for biomarker determination by 2 independent expert echocardiographers blinded to biochemical or clinical analysis using either Siemens Acuson X 700 or Mindray 7 ultrasound systems (1.0-4.0MHz). Mean standard echocardiographical indices for diastolic dysfunction (DD) evaluation were then calculated and recorded; operating LV stiffness constant from Doppler early filling deceleration time was also estimated in line with the formula of Garcia et al.,16 assuming a nonrestrictive mitral orifice (LV stiffness constantnonrest = [0.07/EDT],2 with LV stiffness constant = 1.01 LV stiffness constantnonrest − 0.02).
Study DesignAt the time the research was started, clinical and ultrasound parameters of all participants were recorded to classify them as having type I DD (mild, impaired relaxation pattern), II (moderate, pseudonormal pattern with mild to moderate elevation of LV filling pressures) or III/IV DD (reversible/irreversible, severe, restrictive pattern with significant elevation of LV filling pressures) in agreement with the European Association of Echocardiography/American Society of Echocardiography recommendations.17 Patients were studied to analyze whether: a) they showed increased proenzyme concentrations relative to those of controls; b) cpLOX levels correlated with hemodynamic and biochemical study variables; and c) cpLOX incorporated some prognostic information at the 12-month follow-up. The primary composite endpoint was cardiovascular-cause death and/or HF decompensation requiring hospital readmission to relieve severe systemic or pulmonary congestion.
The research protocol was approved by the local institutional Ethics Committee (Hospital Universitario Príncipe de Asturias, Madrid, Spain) under the European Union legislation and World Medical Association Helsinki Declaration (approval CE 05-2012). All participants signed an informed consent form prior to participation in the study. The authors had full access to all the data and all of them agreed to the manuscript as written.
Statistical AnalysisContinuous variables were managed for descriptive and inferential statistics as mean ± standard deviations or median and [interquartile range] when specified and categorical variables as percentages. We used the Student t test and 1-way ANOVA (analysis of variance) with post-hoc Scheffé procedure to compare normally distributed continuous variables, and the Mann–Whitney U and Kruskal-Wallis tests for nonnormally distributed variables tested by the Kolgomorov-Smirnov test. Bivariate associations of the cpLOX concentrations were evaluated by means of Pearson or Spearman correlations as indicated. Then, for adjusted multivariate lineal regression analysis (Wald), cpLOX was used as the dependent variable, with backward elimination of the other explanatory variables, until the 4 most significantly related biomarkers were selected. Once biomarker concentrations were dichotomized at their median value, the unadjusted association of each potential prognostic biomarker with the clinical outcome was assessed by using a time to-event analysis with univariable Cox proportional hazards regressions (median cpLOX cutoff level = 38.20 ng/mL). Next, a multivariate Cox model was developed with those biomarkers, demonstrating a P value threshold of .05 in the univariate analysis. After adjustment for age, sex, NYHA functional class, the presence of ≥ 3 comorbidities (including all those constituent of the metabolic syndrome, chronic cerebro/peripheral vascular disease, atrial fibrillation and chronic obstructive lung disease/obstructive sleep apnea-hypopnea syndrome), Kansas City Cardiomyopathy Questionnaire score, estimated glomerular filtration rate, DD grade, E/e’, and pulmonary artery systolic pressure, a resultant statistical model was obtained. This model included cpLOX and the 2 most significant biomarkers (BNP and galectin-3) out of all the predictor biomarkers tested in the univariate analysis and was constructed by using a stepwise procedure. Thus, the model required a minimum of 10 events per each selected variable for an accurate regression estimate.18 The Kaplan-Meier method was used to measure the 12-month overall survival and the log-rank test to assess the significance of the comparison between tertiles of cpLOX concentrations. Differences of at least P < .05 (2-sided) were accepted as significant. All calculations were made by using a SPSS 14.0 statistical software package (SPSS Inc, Chicago, Illinois, United States).
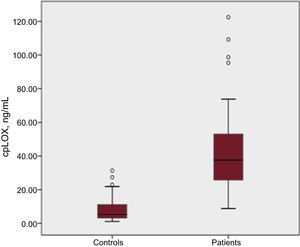
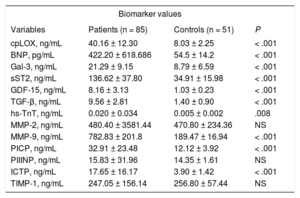
RESULTSBaseline CharacteristicsGeneral clinical data, echocardiographic and biochemical parameters of interest are summarized in Table 1, Table 2, Table 3, and Table 4. It should be emphasized that our patients–especially those with more advanced DD (types II-IV) –showed unequivocally increased cpLOX levels compared with controls (cpLOX: 40.16 ± 12.30 ng/mL vs 8.03 ± 2.25 ng/mL; P < .001) (Figure 1).
General Characteristics of the Patient Group
| General characteristics | Values (n = 85) | General characteristics | Values (n = 85) |
|---|---|---|---|
| Age, y | 79.6 ± 9.5 | Body mass index | 29.4 ± 6.5 |
| Sex, % female | 55.2 | Hb, mg/dL | 12.7 ± 2.1 |
| Metabolic syndrome, % | 58.8 | Transferrin saturation, % | 21.1 ± 2.8 |
| COPD (GOLD I-II)/OSAHS, % | 25.8 | Creatinine, μmol/L | 142 ± 87 |
| Arterial hypertension, % | 97.6 | HbA1c | 6.6 ± 1.3 |
| Dyslipidemia, % | 41.1 | LDL-C, mmol/L | 3.6 ± 0.8 |
| DM (with or without MS), % | 57.6 | TSH, mU/mL | 2.97 ± 2.81 |
| ≥ 2 comorbidities, % | 94.1 | eGFR | 52.5 ± 19.2 |
| Smoking antecedent, % | 38.8 | Advanced DD (grades II-IV), % | 62.3 |
| Stroke antecedent, % | 8.2 | Atrial fibrillation, % | 49.4 |
| Mild peripheral vascular disease, % | 2.3 | QRS >120 msec, % | 34.1 |
| Beta-blockers, % | 48.8 | II/III mitral regurgitation, % | 25.8 |
| ACE inhibitors/ARB/MRA, % | 91.7 | NYHA class III-IV, % | 48.2 |
| Thiazides/loop diuretics, % | 94.1 | KCCQ test score | 41.5 ± 17.7 |
| Catheter ablation procedure, % | 3.5 | Mortality (1 y), % | 11.7 |
| Cardiac pacemaker, % | 9.4 | Nonfatal HF hospitalization (1 y), % | 12.9 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; DD, diastolic dysfunction; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; GOLD, Global Initiative for Chronic Obstructive Lung Disease; Hb, hemoglobin; HbA1c, glycosylated hemoglobin; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; LDL-C, low-density lipoprotein cholesterol; MRA, mineralocorticoid receptor antagonist; MS, metabolic syndrome; NYHA, New York Heart Association; OSAHS, obstructive sleep apnea-hypopnea syndrome; TSH, thyroid stimulating hormone.
Unless otherwise indicated, data are expressed as mean ± standard deviation.
Echographic Indexes Within Each Hemodynamic Subgroup
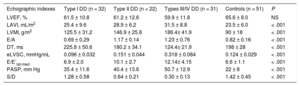
| Echographic indexes | Type I DD (n = 32) | Type II DD (n = 22) | Types III/IV DD (n = 31) | Controls (n = 51) | P |
|---|---|---|---|---|---|
| LVEF, % | 61.5 ± 10.8 | 61.2 ± 12.6 | 59.9 ± 11.8 | 65.6 ± 8.0 | NS |
| LAVI, mL/m2 | 25.4 ± 9.6 | 28.9 ± 6.2 | 31.5 ± 8.8 | 23.5 ± 6.0 | < .001 |
| LVMI, g/m2 | 125.5 ± 31.2 | 146.9 ± 25.8 | 186.4± 41.9 | 90 ± 18 | < .001 |
| E/A | 0.69 ± 0.29 | 1.17 ± 0.14 | 1.23 ± 0.76 | 0.82 ± 0.16 | < .001 |
| DT, ms | 225.8 ± 50.6 | 180.2 ± 34.1 | 124.4± 21.9 | 198 ± 28 | < .001 |
| eLVSC, mmHg/mL | 0.096 ± 0.032 | 0.151 ± 0.044 | 0.318 ± 0.084 | 0.124 ± 0.029 | < .001 |
| E/E’lat-med | 6.9 ± 2.0 | 10.1 ± 2.7 | 12.14± 4.15 | 6.6 ± 1.1 | < .001 |
| PASP, mm Hg | 35.4 ± 11.6 | 40.4 ± 13.6 | 50.7 ± 12.9 | 22 ± 8 | < .001 |
| S/D | 1.28 ± 0.58 | 0.64 ± 0.21 | 0.30 ± 0.13 | 1.42 ± 0.45 | < .001 |
A, diastolic late peak transmitral flow wave velocity; DD, diastolic dysfunction; DT, deceleration time; E, diastolic early peak transmitral flow wave velocity; E/E’lat-med, left ventricular filling index from lateral and medial mitral valve annulus tissular Doppler mean velocities; eLVSC, estimated left ventricular stiffness constant; LAVI, left atrium volume index; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; PASP, pulmonary artery systolic pressure; S/D, ratio of systolic to diastolic wave velocities in pulmonary venous flow.
Data are expressed as mean ± standard deviation.
Values of cpLOX, Profibrogenic Cytokines, Hormones, and Collagen Turnover Biomarkers in the Entire Patient Group
| Biomarker values | |||
|---|---|---|---|
| Variables | Patients (n = 85) | Controls (n = 51) | P |
| cpLOX, ng/mL | 40.16 ± 12.30 | 8.03 ± 2.25 | < .001 |
| BNP, pg/mL | 422.20 ± 618.686 | 54.5 ± 14.2 | < .001 |
| Gal-3, ng/mL | 21.29 ± 9.15 | 8.79 ± 6.59 | < .001 |
| sST2, ng/mL | 136.62 ± 37.80 | 34.91 ± 15.98 | < .001 |
| GDF-15, ng/mL | 8.16 ± 3.13 | 1.03 ± 0.23 | < .001 |
| TGF-β, ng/mL | 9.56 ± 2.81 | 1.40 ± 0.90 | < .001 |
| hs-TnT, ng/mL | 0.020 ± 0.034 | 0.005 ± 0.002 | .008 |
| MMP-2, ng/mL | 480.40 ± 3581.44 | 470.80 ± 234.36 | NS |
| MMP-9, ng/mL | 782.83 ± 201.8 | 189.47 ± 16.94 | < .001 |
| PICP, ng/mL | 32.91 ± 23.48 | 12.12 ± 3.92 | < .001 |
| PIIINP, ng/mL | 15.83 ± 31.96 | 14.35 ± 1.61 | NS |
| ICTP, ng/mL | 17.65 ± 16.17 | 3.90 ± 1.42 | < .001 |
| TIMP-1, ng/mL | 247.05 ± 156.14 | 256.80 ± 57.44 | NS |
BNP, B-type natriuretic peptide; cpLOX, circulating prolysyl oxidase; ICTP, carboxi-terminal telopeptide of collagen I; Gal-3, galectin-3; GDF-15, growth differentiaton factor-15; hs-TnT, high-sensitivity troponin T; MMP, matrix metalloproteinase; PICP, carboxi-terminal propeptide of procollagen I; PIIINP, amino-terminal propeptide of procollagen III; sST2, soluble toll-like/interleukin-1 type-II receptor; TGF-β, transforming growth factor beta; TIMP-1, tissue inhibitor of metalloproteinase-1.
Data are expressed as mean ± standard deviation.
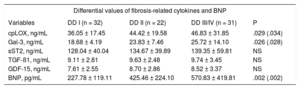
Values of Fibrosis-related Cytokines and B-type Natriuretic Peptide Within Each Hemodynamic Subgroup
| Differential values of fibrosis-related cytokines and BNP | ||||
|---|---|---|---|---|
| Variables | DD I (n = 32) | DD II (n = 22) | DD III/IV (n = 31) | P |
| cpLOX, ng/mL | 36.05 ± 17.45 | 44.42 ± 19.58 | 46.83 ± 31.85 | .029 (.034) |
| Gal-3, ng/mL | 18.68 ± 4.19 | 23.83 ± 7.46 | 25.72 ± 14.10 | .026 (.028) |
| sST2, ng/mL | 128.04 ± 40.04 | 134.67 ± 39.89 | 139.35 ± 59.81 | NS |
| TGF-ß1, ng/mL | 9.11 ± 2.81 | 9.63 ± 2.48 | 9.74 ± 3.45 | NS |
| GDF-15, ng/mL | 7.61 ± 2.55 | 8.70 ± 2.86 | 8.52 ± 3.37 | NS |
| BNP, pg/mL | 227.78 ± 119.11 | 425.46 ± 224.10 | 570.83 ± 419.81 | .002 (.002) |
BNP, B-type natriuretic peptide; cpLOX, circulating prolysyl oxidase; DD, diastolic dysfunction; Gal-3, galectin-3; GDF-15, growth differentiation factor-15; sST2, soluble toll-like/interleukin-1 type-II receptor; TGF-β, transforming growth factor beta.
Data are expressed as mean ± standard deviation.
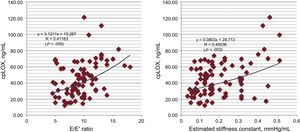
We confirmed positive Pearson direct correlations of weak significance between cpLOX and variables reflecting elevated LV filling pressures (E/E’ ratio) and myocardial stiffness (estimated LV stiffness constant) in the entire HM-HFpEF group (respectively, R = 0.411; P = .005, and R = 0.450; P = .003) (Figure 2).
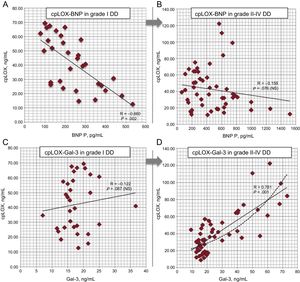
Concerning cpLOX associative analysis, the proenzyme levels demonstrated direct Pearson correlations with galectin-3, soluble toll-like/interleukin-1 type-II receptor, transforming growth factor beta, carboxi-terminal propeptide of procollagen I and BNP (data not shown), although only galectin-3 proved to hold a significant independent relationship (β = 0.688; P < .001) in an adjusted model where BNP also appeared to be negatively correlated (Table 5A). In relation to independent correlations of cpLOX within each hemodynamic subgroup, only a few of them were relevant in the multivariate analysis (Table 5B). Briefly, in the type I DD subgroup, cpLOX was negatively correlated with BNP (β = –0.660, P = .002) but not with Gal-3 (Figure 3A and Figure 3B). In contrast, in the group of patients with grades II to IV DD, only galectin-3 showed a substantial positive link with cpLOX (β = 0.781; P < .001) (Figure 3C and Figure 3D). The other biomarkers did not show significant associations with cpLOX.
Results of cpLOX Pathophysiological Assessment in the Entire Cohort (A) and Within Each Subgroup of Diastolic Dysfunction (B)
| A. Overall multivariate lineal regression analysis (n = 85) | ||
|---|---|---|
| Variables | β | P |
| cpLOX-Gal-3 | 0.688 | < .001 |
| cpLOX-sST2 | 0.243 | .050 |
| cpLOX- TGF-ß1 | 0.214 | .068 (NS) |
| cpLOX- GDF-15 | 0.137 | .125 (NS) |
| cpLOX-BNP | –0.401 | .019 |
| B. Differential multivariate lineal regression analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | DD I (n = 32) | DD II (n = 22) | DD III/IV (n = 31) | DD II/IV (n = 53) | ||||
| β | P | β | P | β | P | β | P | |
| cpLOX-Gal-3 | 0.122 | NS | 0.661 | .005 | 0.910 | < .001 | 0.781 | <.001 |
| cpLOX-sST2 | 0.203 | NS | 0.200 | NS | 0.317 | .040 | 0.238 | NS |
| cpLOX-TGF-ß1 | 0.146 | NS | 0.157 | NS | 0.313 | .041 | 0.230 | NS |
| cpLOX-GDF-15 | 0.096 | NS | 1.304 | NS | 1.569 | NS | 0.145 | NS |
| cpLOX-BNP | –0.660 | .002 | –0.176 | NS | -0.101 | NS | –0.158 | NS |
BNP, B-type natriuretic peptide; cpLOX, circulating prolysyl oxidase; DD, diastolic dysfunction; Gal-3, galectin-3; GDF-15, growth differentiaton factor-15; sST2, soluble toll-like/interleukin-1 type-II receptor; TGF-β, transforming growth factor beta.
cpLOX pathophysiological assessment within each DD subgroup (multivariate analysis of selected biomarker values in patients with diastolic dysfunction grade I compared with those with grades II to IV). A and B: correlations between cpLOX and BNP; C and D: correlations between cpLOX and galectin-3; exponential trend in grades II to IV is also depicted (dotted line). BNP, B-type natriuretic peptide; cpLOX, circulating prolysyl oxidase; DD, diastolic dysfunction; Gal-3, galectin-3.
At the end of the 1-year follow-up period, 21 patients (24.7%) were rehospitalized for acute HF decompensation at least once and later discharged, and 10 more (11.7%) died from cardiovascular causes. Kaplan-Meier survival analysis among participants separated into tertiles of cpLOX concentrations showed significant mortality rate differences at 12 months when cpLOX levels exceeded 52.20 ng/mL (results not shown) (log rank = 4.45; P = .034). Therefore, this cutoff value can be used as a modest but recognizable discriminator of mortality risk. Nevertheless, after adjustment for clinical, biochemical and ultrasound variables, Cox proportional hazards regression analysis revealed that cpLOX had an independent prognostic value as part of an optimized model including BNP and galectin-3 as the most clear-cut copredictor biomarkers at 12 months (cpLOX hazard ratio [HR], 1.360; 95% confidence interval [95%CI], 1.126-1.638, P = .046; Gal-3 HR, 1.923; 95%CI, 1.022-2.853, P = .009; BNP HR, 2.976; 95%CI, 1.210-4.216, P < .001].
DISCUSSIONGeneral Overview on Myocardial Lysyl Oxidase in Hypertensive-metabolic Heart Failure With Preserved Ejection FractionIn the absence of information about circulating LOX in the clinical domain of HFpEF, our results confirmed a marked increase in proenzyme levels in this condition; this rise was more prominent if advanced DD was present. As a whole, these figures are fully compatible with previous molecular observations of high LOX expression in the myocardial tissue of hypertensive patients19 and they also reinforce the concept that a LOX-catalyzed fibrotic process driven by different hormones and cytokines is one of the pivotal facts in HM-HFpEF development.20–23 Nonetheless, reservations must be expressed about LOX overexpression and collagen I deposition as the unique LOX-mediated mechanism of poor cardiovascular compliance in different pathological circumstances. For instance, Chen et al.24 reported that high arterial stiffness indices in obese patients translated into decreased absolute serum levels of LOX, suggesting that excessive expression of several products and oxidative stress typically observed in asymptomatic obese patients may cause a lack of LOX maturation, accompanied by elastin fragmentation and loss of arterial distensibility. Accordingly, there seems to be an equilibrium between stiff collagen I deposition and distensible elastin formation in physiological situations. Therefore, we assume that not only certain cardiovascular conditions, ie, dilated cardiomyopathy, coronary allograft restenosis,25 but also HM-HFpEF, might result from LOX upregulation, leading to accumulation or absolute excess of rigid collagen I. However, in other disorders, for example, severe aortic stiffness in obese patients without symptomatic heart failure, the loss of vascular distensibility may arise from enzyme underexpression, causing poor elastin production and relative collagen I abundance.
Potential Mechanisms of Myocardial Lysyl Oxidase Humoral Control in Hypertensive-metabolic Heart Failure With Preserved Ejection FractionFrom previous work demonstrating the presence of inflammatory cells in failing human myocardium, it has been generally admitted that collagen fibrosis involves the response to specific physical-chemical stimuli mediated by hormones and cytokines.26 However, little is known about specific mechanisms of in vivo myocardial LOX humoral regulation because most studies on LOX physiology were conducted at histochemical and molecular levels or as a result of experiments performed in a mouse atrial cardiomyocyte tumor lineage.27 Therefore, the notable cpLOX-galectin-3 correlation we found suggests the possible existence of a galectin-3 dependent humoral stimulating mechanism of cardiac LOX transcription signaling pathways, in agreement with what was already demonstrated for the LOX-2 homologue in experimental animals.28 Furthermore, considering that vascular macrophages are the main source of galectin-3 and that T lymphocyte-macrophage depleted mice lack LOX activity,29 to explain our findings, we can uphold that galectin-3 plays a key role in cardiac LOX synthesis regulation. In a finalistic manner, we can even speculate that the notable independent negative association of BNP with cpLOX we detected in cases with a simple impairment of myocardial relaxation might reflect the antifibrotic BNP effects to counteract supranormal plasma levels of fibrogenic hormones and cytokines.30
In summary, our findings are consistent with the following: a) the formation of organized collagen I originates from the onset of DD despite BNP effects; b) galectin-3, more than any other cytokine, may have a critical influence on LOX-mediated heart fibrosis as hemodynamic deterioration progresses; and c) mechanistically, BNP as well as galectin-3 could have opposing regulatory effects on cardiac LOX throughout the natural course of HM-HFpEF. In other words, we could assume that myocardial stiffness–an essential hallmark in HM-HFpEF31—might depend on an early cross-linking of collagen I, which becomes more relevant when galectin-3-mediated LOX upregulation prevails.
Circulating Prolysyl Oxidase Prognostic Value in Hypertensive-metabolic Heart Failure With Preserved Ejection FractionThe modest accuracy of cpLOX as a biomarker to provide significant prognostic information compared with other cardiac biomarkers32 limits the usefulness of its determination in clinical practice. Nevertheless, this lack of strong predictive value in HFpEF indirectly stresses that heart rigid fibrosis—as presumed from cpLOX values—appears to be in progress from the relatively benign type I HM-HFpEF to the final and most severe DD stages. However, since cpLOX concentrations > 52.2 ng/mL were associated with increased mortality at 12 months in our series, it is not contradictory to hypothesize that the higher enzyme levels are, the more serious the compromise of diastolic function, leading to a more negative overall prognosis. Whether or not cpLOX could become a useful prognostic biomarker in HM-HFpEF will have to be detected through area under the curve comparative analysis (ie, on the c-statistic basis) with future research involving larger cohorts of patients.
LimitationsThis work has clear limitations, although they are similar to those of any single-center, descriptive, observational study. First, we cannot exclude unmeasured confounding variables as an alternative explanation for the results obtained. Also, our relatively small-sized samples make it difficult to draw firm conclusions about the study objectives. Second, although we made efforts to assess only stable individuals without typical fibrotic diseases, many of them were elderly people with noncritical comorbidities who might have a profibrotic milieu (eg, atrial fibrillation, chronic cerebrovascular/asymptomatic peripheral artery disease, nonadvanced renal failure, osteoartrosis). Therefore, our findings should be interpreted with caution. Finally, we were unable to compare proenzyme concentrations in non-HM-HFpEF patients and, consequently, upcoming studies with a larger number of more diverse samples are required to assess cpLOX in other HFpEF phenotypes.
CONCLUSIONSPutative overexpression of myocardial LOX in stage C, nonischemic, garden-variety HM-HFpEF is accompanied by high cpLOX levels, especially when patients have advanced DD. These levels are moderately correlated with estimated LV filling pressures and myocardial stiffness and they have some clinical predictive value at 12 months. According to our associative analysis model, LOX activity in cardiac fibrosis process might be mainly driven by galectin-3 when increased LV filling pressure is evident. In addition, our results suggest that there might be a BNP-dependent mechanism of LOX counter-regulation in HM-HFpEF. However, this will eventually prove insufficient to prevent the galectin-3 induced steady collagen I accumulation. Regardless of how far-reaching the implications of our findings may be, it has to be emphasized that these physiopathological considerations are rational hypotheses derived from observations that need to be confirmed in upcoming studies in larger cohorts and with additional research at the molecular level. In the same vein, we believe that both BNP and galectin-3 deserve special attention as opposite regulators of enzyme activity throughout the natural history of HM-HFpEF and as specific targets for antifibrotic pharmacological intervention in the near future.
FUNDINGThis study was supported by a grant from Fundación para la Investigación Biomédica del Hospital Universitario Príncipe de Asturias (grant FIB-PI09-03).
CONFLICTS OF INTERESTNone declared.
- -
Interstitial myocardial fibrosis is a key process accompanying HFpEF; collagen fiber arrangement results from the action of LOX; this enzyme is highly expressed in the myocardial tissue of hypertensive patients.
- -
The LOX concentrations are almost negligible in the blood of healthy participants; no information exists on plasma LOX in HFpEF patients.
- -
The LOX upregulation is known to happen in certain animal models and human cardiovascular conditions (ie, experimental heart hypertrophy, dilated cardiomyopathy, coronary allograft restenosis, atrial fibrillation).
- -
Galectin-3 stimulates transcription signaling pathways of LOX-2 homologue in experimental animals.
- -
Nonischemic, garden-variety HM-HFpEF is accompanied by high cpLOX levels.
- -
The cpLOX correlates with ultrasound-estimated LV filling pressures and myocardial stiffness parameters.
- -
In HFpEH patients, high cpLOX levels are inversely correlated with BNP in those with lower grades of DD, and positively with galectin-3 if a restrictive pattern of DD ensues.
- -
Although not clinically relevant, cpLOX levels seem to involve some predictive value at 12 months in HFpEF patients.
.